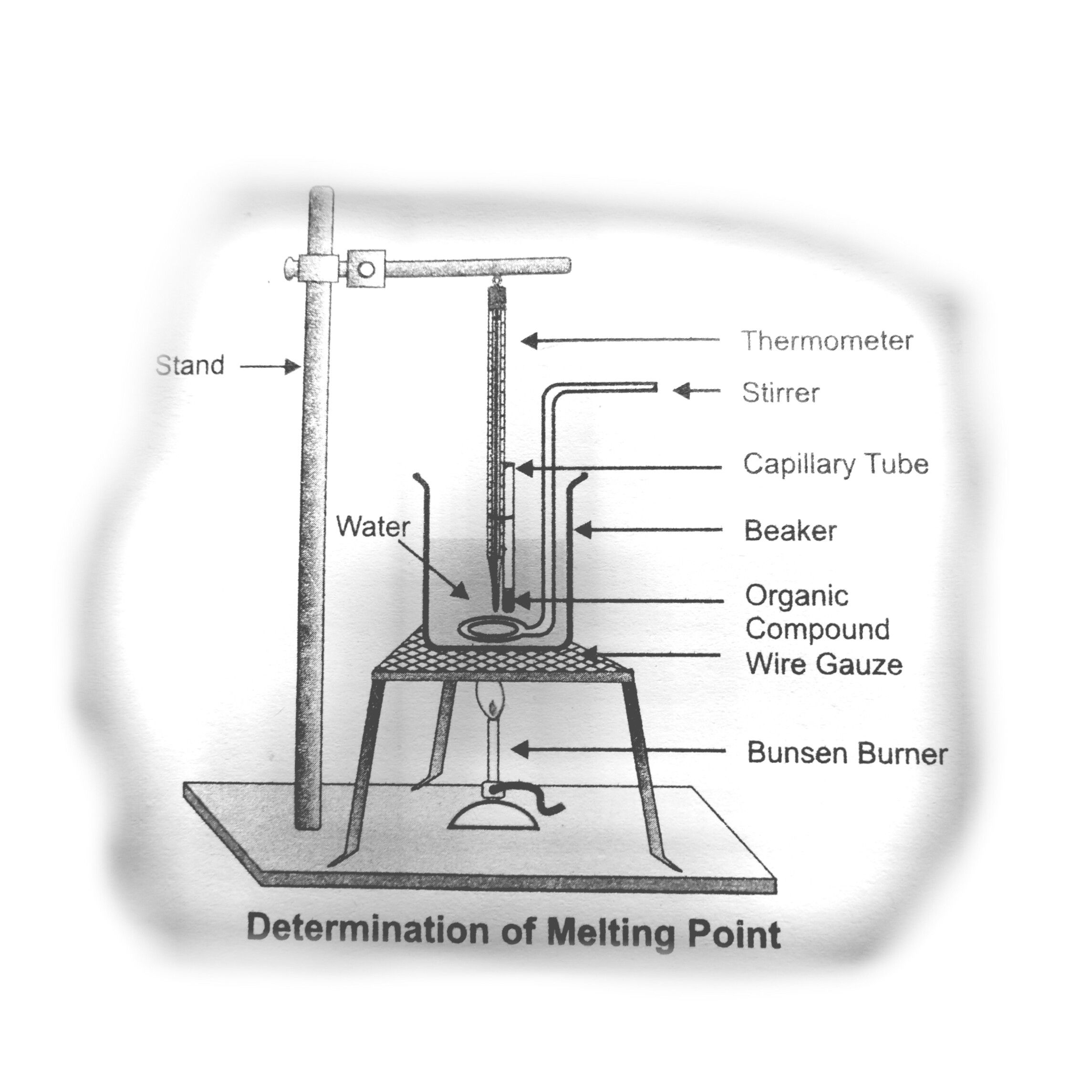
At The Melting Point The Solid Form Of A Substance
At The Melting Point The Solid Form Of A Substance - At that temperature, the solid and liquid states of the substance are in equilibrium. The liquid form of the substance. Web the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of the solid or the freezing point of the liquid. To describe the solid, liquid, and gas phases of matter. Solid substance a has a melting point of 100 degrees celsius. Molecular solids composed of molecules with permanent dipole. Substances in the molten state. This is the point at which both liquid and solid phase. Water can take many forms. Web melting point (mp) is a quick and easy analysis that may be used to qualitatively identify relatively pure samples (approximately <10% impurities).
At low temperatures (below 0 o c ), it is a solid. Molecular solids composed of molecules with permanent dipole. Web metals tend to have high melting points, though notable exceptions are mercury, which has a melting point of minus 37.84 degrees fahrenheit (minus 38.8. The liquid form of the substance. Web melting point (o c) boiling point (o c) agate: Web the melting point of a solid is the same as the freezing point of the liquid. At that temperature, the solid and liquid states of the substance are in equilibrium. Web science chemistry chemistry questions and answers at the melting point, the solid form of a substance choose. Web melting of a pure solid occurs at a higher temperature than melting of an impure solid, a concept called melting point depression (or freezing point depression). Water can take many forms.
At low temperatures (below 0 o c ), it is a solid. Solid substance a has a melting point of 100 degrees celsius. Web substances consisting of larger, nonpolar molecules have larger attractive forces and melt at higher temperatures. Molecular solids composed of molecules with permanent dipole. Web melting point (mp) is a quick and easy analysis that may be used to qualitatively identify relatively pure samples (approximately <10% impurities). The liquid form of the substance. This is the point at which both liquid and solid phase. The melting point is a physical property of a solid and can be used to help. Web melting of a pure solid occurs at a higher temperature than melting of an impure solid, a concept called melting point depression (or freezing point depression). Substances in the molten state.
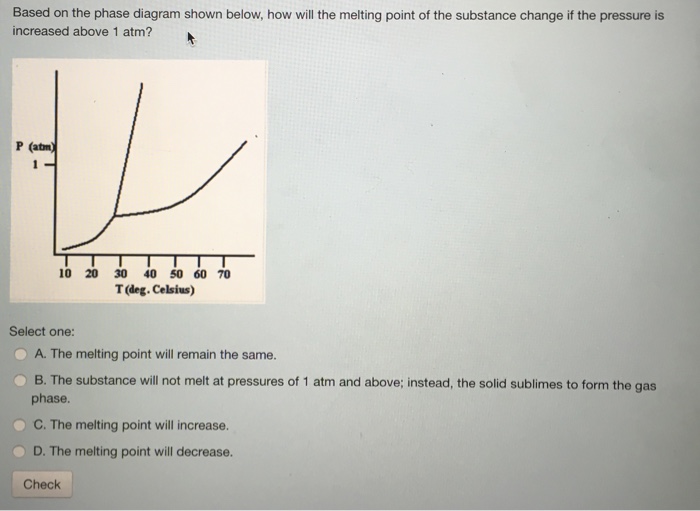
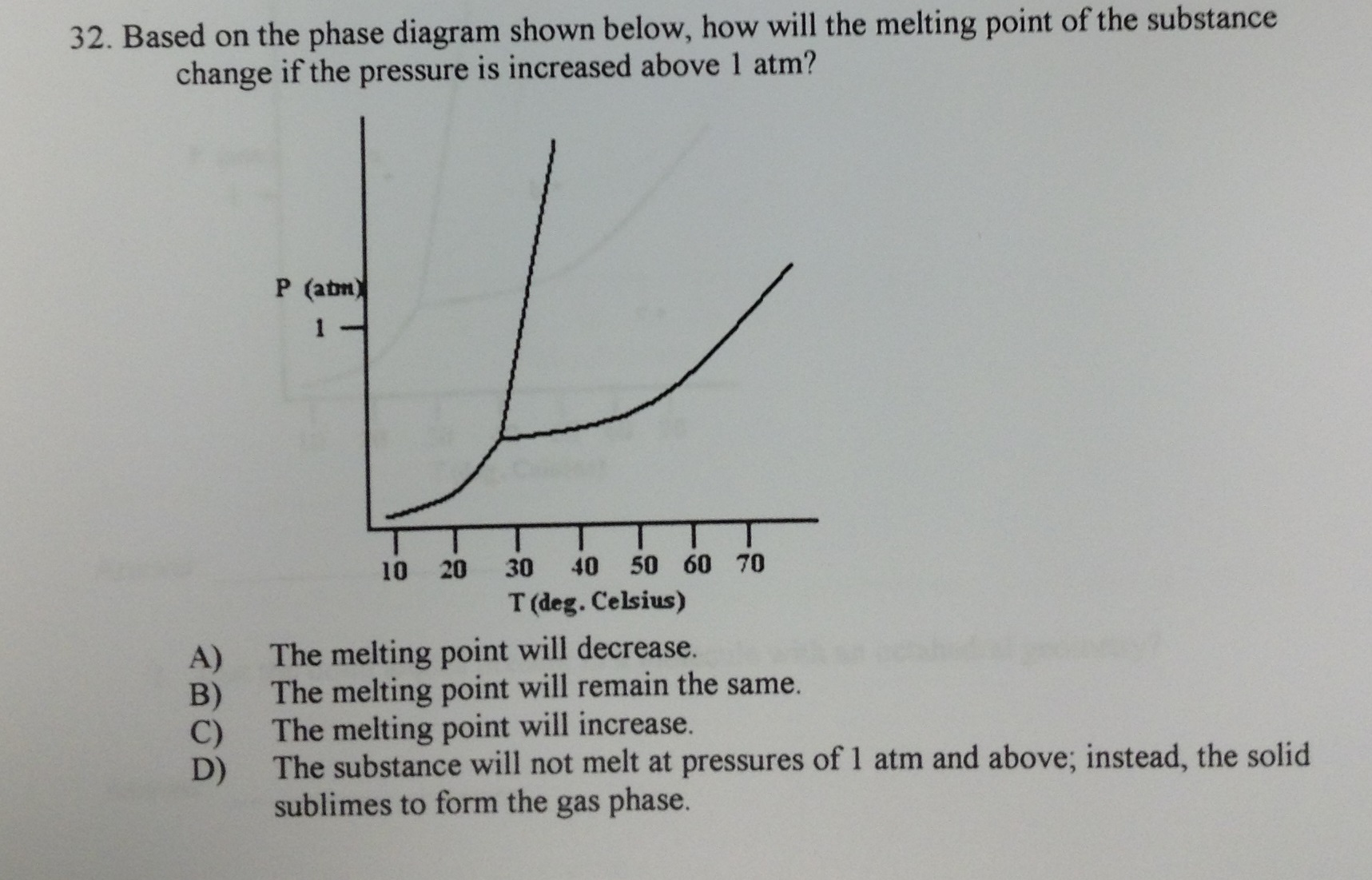
Solved Based On The Phase Diagram Shown Below, How Will T...
This is the point at which both liquid and solid phase. Web metals tend to have high melting points, though notable exceptions are mercury, which has a melting point of minus 37.84 degrees fahrenheit (minus 38.8. Web the temperature at which a solid melts is known as the melting point (mp) of that substance. Web relatively high melting point: Web.
Solved Based On The Phase Diagram Shown Below, How Will T...
Web the temperature at which a solid melts is known as the melting point (mp) of that substance. Web melting of a pure solid occurs at a higher temperature than melting of an impure solid, a concept called melting point depression (or freezing point depression). Water can take many forms. Web melting point (mp) is a quick and easy analysis.
Laurie Media Melting Point Of Ice
Web science chemistry chemistry questions and answers at the melting point, the solid form of a substance choose. Web at the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid. Web melting of a pure solid occurs at a higher temperature than melting.
Solved Suppose a block of a substance has mass 5.6 kg and is
To describe the solid, liquid, and gas phases of matter. Water can take many forms. Web the melting point of a solid is the same as the freezing point of the liquid. Substances in the molten state. Web metals tend to have high melting points, though notable exceptions are mercury, which has a melting point of minus 37.84 degrees fahrenheit.
PPT Physical and Chemical Properties 9/16/2014 PowerPoint
Web at the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid. Web melting of a pure solid occurs at a higher temperature than melting of an impure solid, a concept called melting point depression (or freezing point depression). Solid substance a has.
Paul's Science Lab freezing and melting points
Web the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of the solid or the freezing point of the liquid. Web melting point (o c) boiling point (o c) agate: Substances in the molten state. The liquid form of the substance. Web at the melting point, the ordering.
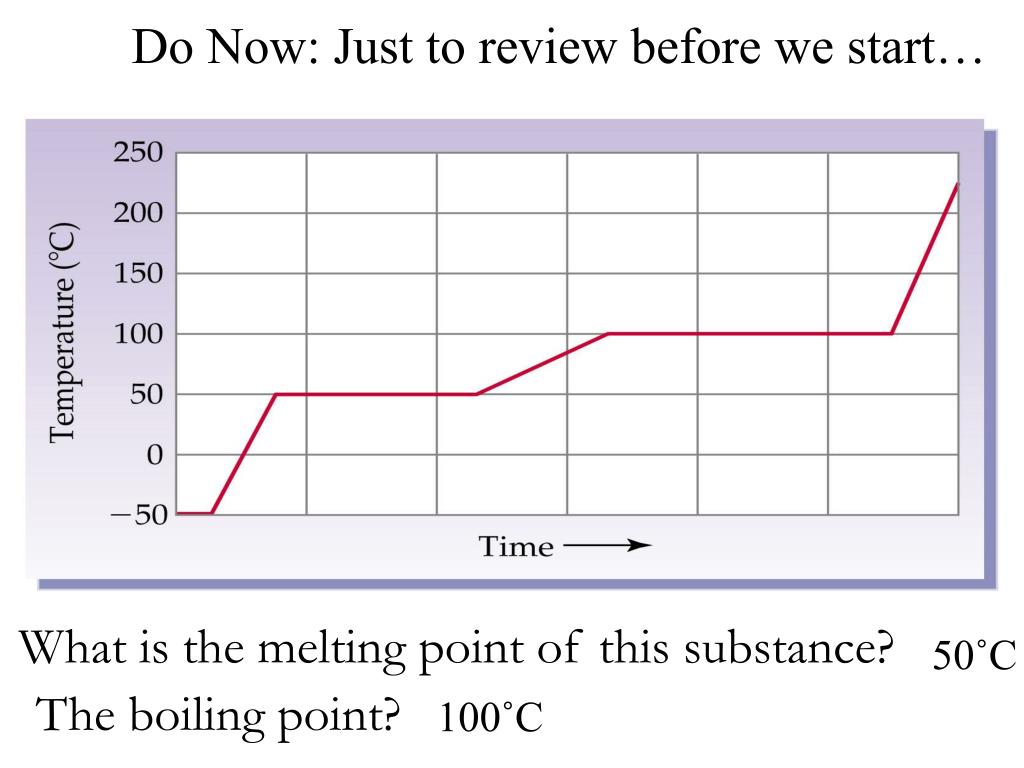
PPT What is the melting point of this substance? PowerPoint
Web the temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Substances in the molten state. At low temperatures (below 0 o c ), it is a solid. Web the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting.
How to measure the melting point of a substance YouTube
Web relatively high melting point: At that temperature, the solid and liquid states of the substance are in equilibrium. The melting point is a physical property of a solid and can be used to help. Web at the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts.
Experiment of physical States of Matter Determine the melting point of
Solid substance a has a melting point of 100 degrees celsius. Web the temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. Web the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of the solid or the.
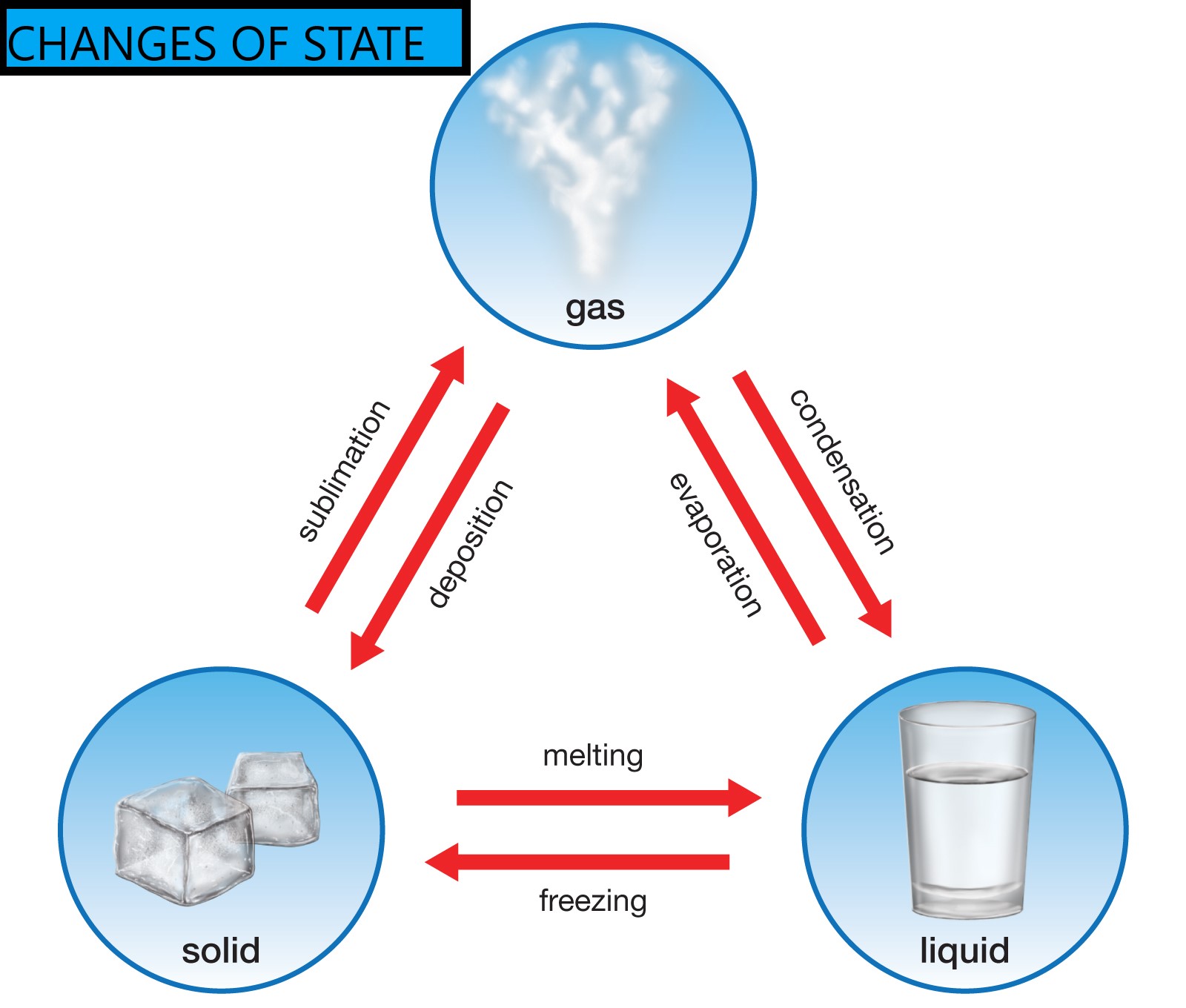
El blog de juanmateacher 6º CHANGES OF STATE OF MATTER
Web melting point (o c) boiling point (o c) agate: Solid substance a has a melting point of 100 degrees celsius. Substances in the molten state. Web melting point (mp) is a quick and easy analysis that may be used to qualitatively identify relatively pure samples (approximately <10% impurities). At low temperatures (below 0 o c ), it is a.
Web The Temperature At Which Solid Changes Its State To Liquid At Atmospheric Pressure Is Called The Melting Point Of That Liquid.
This is the point at which both liquid and solid phase. Web at the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid. Web melting point (o c) boiling point (o c) agate: Web science chemistry chemistry questions and answers at the melting point, the solid form of a substance choose.
Web The Melting Point Of A Solid Is The Same As The Freezing Point Of The Liquid.
Web substances consisting of larger, nonpolar molecules have larger attractive forces and melt at higher temperatures. Web the temperature at which the solid and liquid phases of a given substance are in equilibrium is called the melting point of the solid or the freezing point of the liquid. The melting point is a physical property of a solid and can be used to help. The liquid form of the substance.
Solid Substance A Has A Melting Point Of 100 Degrees Celsius.
Web the temperature at which a solid melts is known as the melting point (mp) of that substance. Molecular solids composed of molecules with permanent dipole. Web metals tend to have high melting points, though notable exceptions are mercury, which has a melting point of minus 37.84 degrees fahrenheit (minus 38.8. Substances in the molten state.
Web Melting Point (Mp) Is A Quick And Easy Analysis That May Be Used To Qualitatively Identify Relatively Pure Samples (Approximately <10% Impurities).
To describe the solid, liquid, and gas phases of matter. At that temperature, the solid and liquid states of the substance are in equilibrium. Web melting of a pure solid occurs at a higher temperature than melting of an impure solid, a concept called melting point depression (or freezing point depression). At low temperatures (below 0 o c ), it is a solid.