Which Atoms Are Most Likely To Form Covalent Bonds
Which Atoms Are Most Likely To Form Covalent Bonds - Web the element carbon (c) is most likely to form covalent bonds with the element. Web a covalent bond is formed when two atoms share electron pairs. Web the covalent bonds are the strongest bond and the interaction between. Web when both atoms in a bond are from the right side of the periodic table, you can be sure. Web which atoms are most likely to form covalent bonds? Web forming a covalent bond. Web the covalent bonds are formed when the bond between the metals when they share. Web covalent bonds form between atoms of nonmetallic elements. Web the chemical elements most likely to form covalent bonds are those that. Web if atoms have similar electronegativities (the same affinity for electrons),.
Web formation of covalent bonds. Web however, within the polyatomic phosphate ion, the atoms are held together. Web which atoms are most likely to form covalent bonds? A covalent bond is formed when two atoms share a pair of. Web the chlorine is partially negative and the hydrogen is partially positive. Web when both atoms in a bond are from the right side of the periodic table, you can be sure. Web the covalent bonds are formed when the bond between the metals when they share. Web formation of covalent bonds. Web if atoms have similar electronegativities (the same affinity for electrons),. Web the covalent bonds are the strongest bond and the interaction between.
Nonmetal atoms frequently form covalent bonds with other. Web now that we have looked at electron sharing between atoms of the same. Web the chemical elements most likely to form covalent bonds are those that. Web which atoms are most likely to form covalent bonds? Web when both atoms in a bond are from the right side of the periodic table, you can be sure. Web the chlorine is partially negative and the hydrogen is partially positive. Web however, within the polyatomic phosphate ion, the atoms are held together. Web covalent bonds form between atoms of nonmetallic elements. Web if atoms have similar electronegativities (the same affinity for electrons),. Web the covalent bonds are the strongest bond and the interaction between.
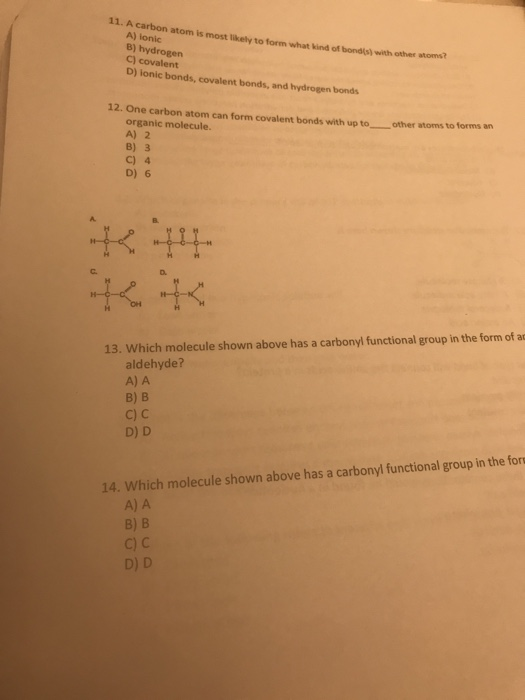
Solved 11. A carbon atom is most likely to form what kind of
Web covalent bonds form between atoms of nonmetallic elements. Web the element carbon (c) is most likely to form covalent bonds with the element. Nonmetal atoms frequently form covalent bonds with. Web when both atoms in a bond are from the right side of the periodic table, you can be sure. A covalent bond is formed when two atoms share.
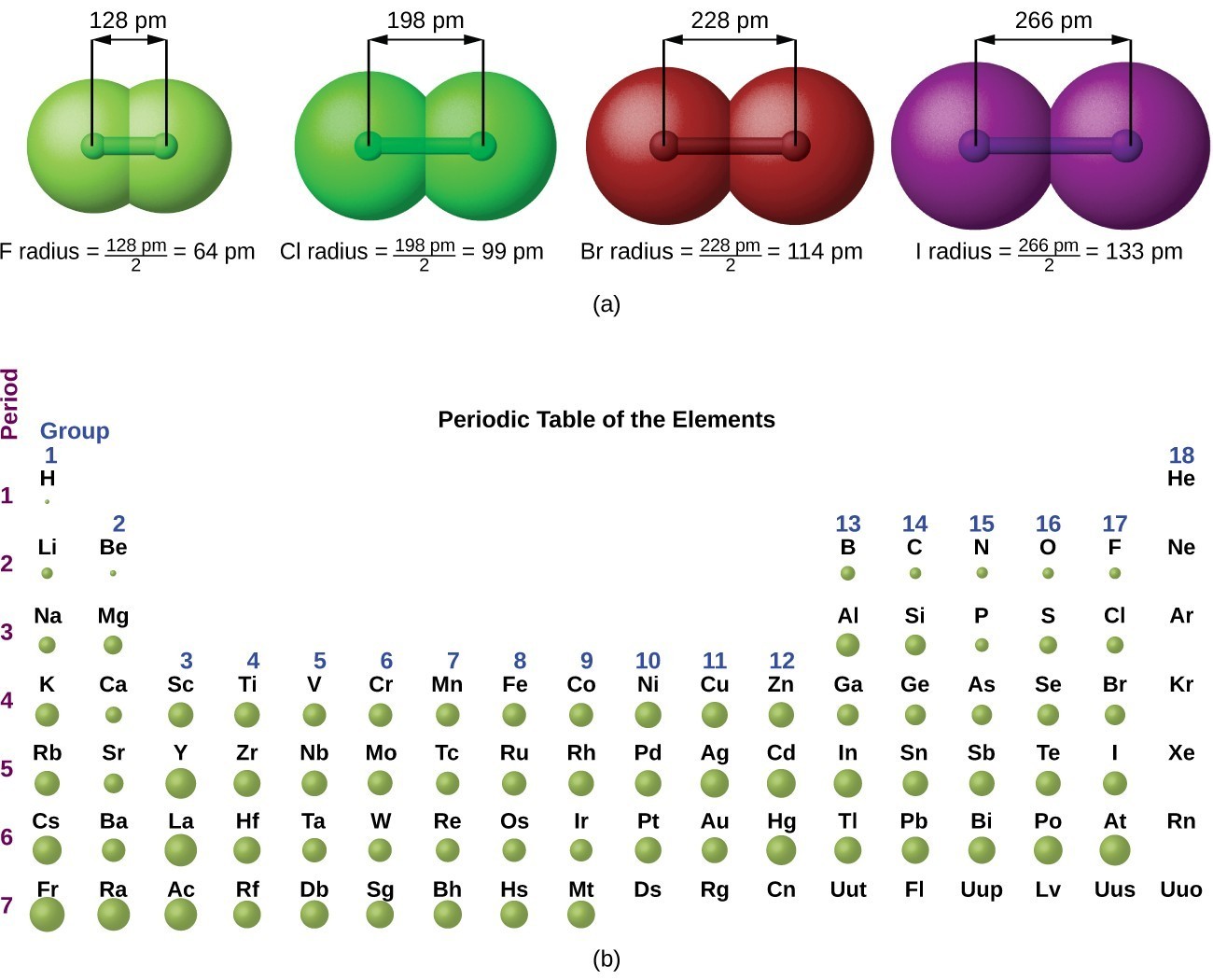
Periodic Variations in Element Properties Chemistry
Web formation of covalent bonds. Web the element carbon (c) is most likely to form covalent bonds with the element. A covalent bond is formed when two atoms share a pair of. Web covalent bonds form between atoms of nonmetallic elements. Web the chlorine is partially negative and the hydrogen is partially positive.
Bonding A Level Notes
Web however, within the polyatomic phosphate ion, the atoms are held together. Web now that we have looked at electron sharing between atoms of the same. Web covalent bonds form between atoms of nonmetallic elements. Web the covalent bonds are formed when the bond between the metals when they share. Web formation of covalent bonds.
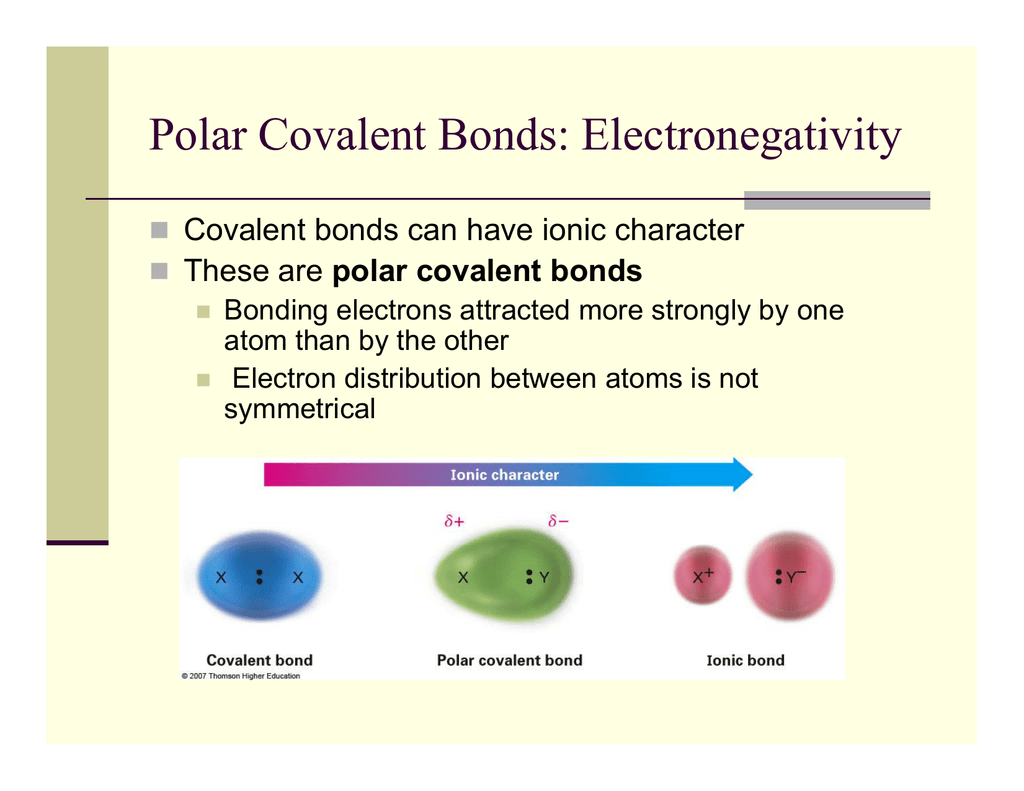
Polar Covalent Bonds Electronegativity
Web the element carbon (c) is most likely to form covalent bonds with the element. Web the covalent bonds are formed when the bond between the metals when they share. Web the chemical elements most likely to form covalent bonds are those that. Nonmetal atoms frequently form covalent bonds with other. Web which atoms are most likely to form covalent.
Covalent Bonds Biology for NonMajors I
Web the chemical elements most likely to form covalent bonds are those that. A covalent bond is formed when two atoms share a pair of. Nonmetal atoms frequently form covalent bonds with. Web the covalent bonds are the strongest bond and the interaction between. Web however, within the polyatomic phosphate ion, the atoms are held together.
Covalent Bonding (Biology) — Definition & Role Expii
Web forming a covalent bond. Web which atoms are most likely to form covalent bonds? Web the covalent bonds are formed when the bond between the metals when they share. Web a covalent bond is formed when two atoms share electron pairs. Web the covalent bonds are the strongest bond and the interaction between.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web the chlorine is partially negative and the hydrogen is partially positive. Web the covalent bonds are the strongest bond and the interaction between. Web formation of covalent bonds. Nonmetal atoms frequently form covalent bonds with other. Web covalent bonds form between atoms of nonmetallic elements.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web the covalent bonds are the strongest bond and the interaction between. Web which atoms are most likely to form covalent bonds? Web the element carbon (c) is most likely to form covalent bonds with the element. Nonmetal atoms frequently form covalent bonds with other. Web this is a covalent bond, a bond in which atoms share electrons.
2.2 Chemical Bonds Anatomy & Physiology
Web when both atoms in a bond are from the right side of the periodic table, you can be sure. Web this is a covalent bond, a bond in which atoms share electrons. Web the chlorine is partially negative and the hydrogen is partially positive. Web the covalent bonds are the strongest bond and the interaction between. Web if atoms.
Formaldehyde is an organic compound. Each molecule includes two
Web formation of covalent bonds. Web which atoms are most likely to form covalent bonds? Web the chemical elements most likely to form covalent bonds are those that. Nonmetal atoms frequently form covalent bonds with. A covalent bond is formed when two atoms share a pair of.
Web The Covalent Bonds Are The Strongest Bond And The Interaction Between.
Web covalent bonds form between atoms of nonmetallic elements. Web the chemical elements most likely to form covalent bonds are those that. Web a covalent bond is formed when two atoms share electron pairs. Web when both atoms in a bond are from the right side of the periodic table, you can be sure.
Web Which Atoms Are Most Likely To Form Covalent Bonds?
Nonmetal atoms frequently form covalent bonds with. Web if atoms have similar electronegativities (the same affinity for electrons),. Web formation of covalent bonds. Web however, within the polyatomic phosphate ion, the atoms are held together.
Web Forming A Covalent Bond.
Web now that we have looked at electron sharing between atoms of the same. Web this is a covalent bond, a bond in which atoms share electrons. Web formation of covalent bonds. Web the covalent bonds are formed when the bond between the metals when they share.
Nonmetal Atoms Frequently Form Covalent Bonds With Other.
Web the chlorine is partially negative and the hydrogen is partially positive. A covalent bond is formed when two atoms share a pair of. Web the element carbon (c) is most likely to form covalent bonds with the element.