When Sodium Atoms Form Sodium Ions They
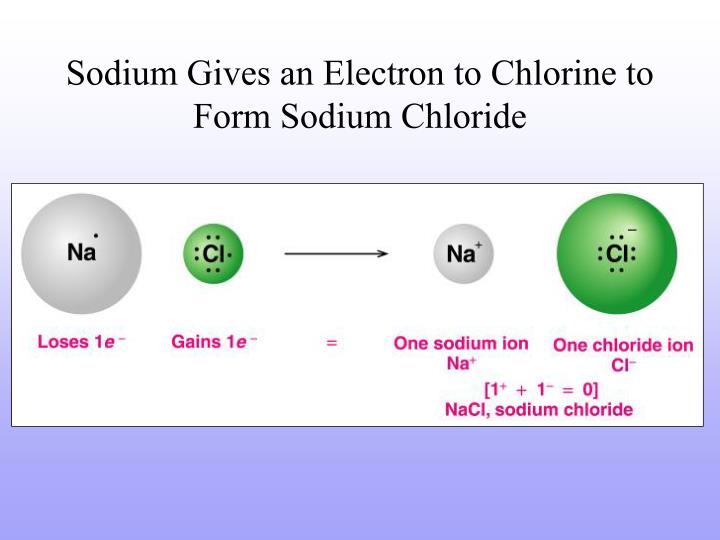
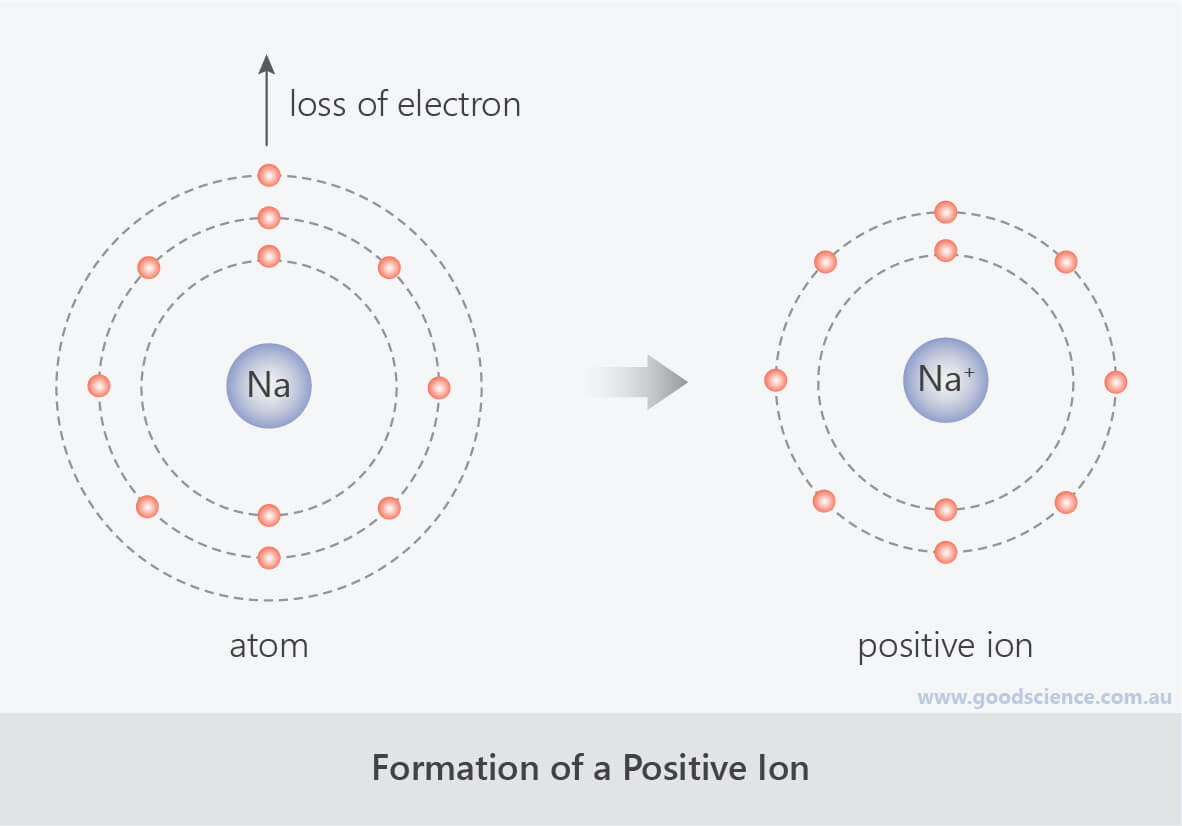
When Sodium Atoms Form Sodium Ions They - Web when sodium atoms form sodium ions, they a. Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. Web when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Web sodium ions are used to build up electrical gradients in the firing of neurons in the brain. In 1807 sir humphry davy became the first to prepare sodium in its elemental form, applying electrolysis to fused sodium hydroxide (naoh). (in chapter 9 “chemical bonds”, we will discuss why atoms form the charges they do.) Its only stable isotope is 23 na. Thus, if you commit the information in table \(\pageindex{1}\) to memory, you will always know what charges most atoms form. Sodium diffuses in and is pumped back out, while potassium does the reverse journey. Web a sodium ion is formed when a sodium atom loses a single electron.
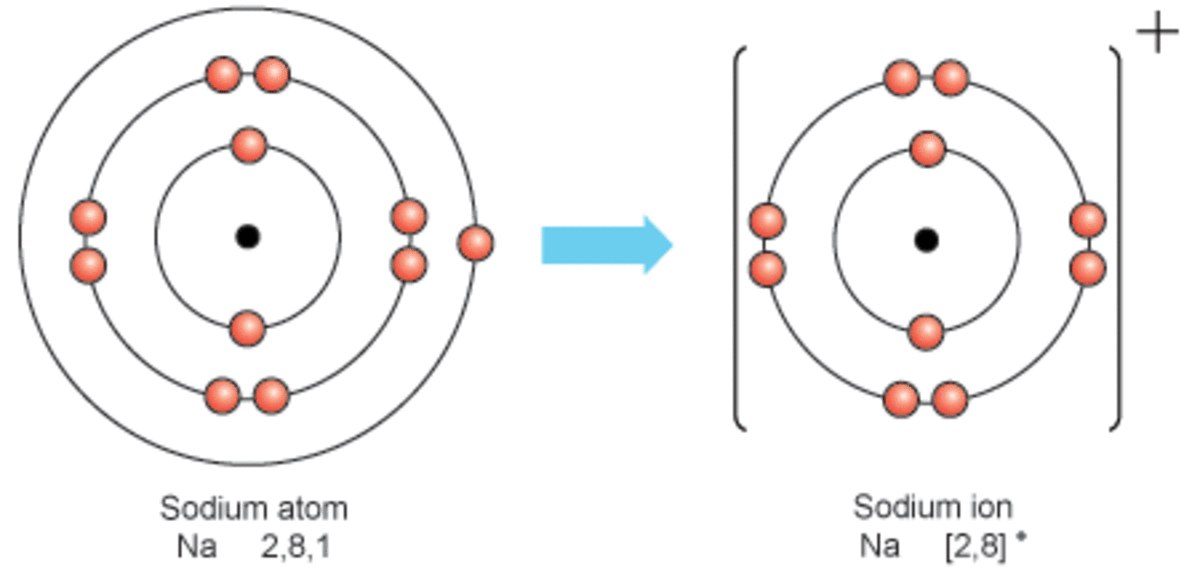
This involves sodium (and its big brother potassium) diffusing through cell membranes. Sodium is an alkali metal, being in group 1 of the periodic table. Web the sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Its only stable isotope is 23 na. It is now the same as that of the noble gas neon. Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. Web when sodium atoms form sodium ions, they a. Thus, if you commit the information in table 3.2 to memory, you will always know what charges most atoms form. The ion is electrically charged, while the atom is electrically neutral. Sodium diffuses in and is pumped back out, while potassium does the reverse journey.
(in chapter 9 “chemical bonds”, we will discuss why atoms form the charges they do.) Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. Thus, if you commit the information in table 3.2 to memory, you will always know what charges most atoms form. Thus, if you commit the information in table \(\pageindex{1}\) to memory, you will always know what charges most atoms form. Web when sodium atoms form sodium ions, they a. In 1807 sir humphry davy became the first to prepare sodium in its elemental form, applying electrolysis to fused sodium hydroxide (naoh). Sodium diffuses in and is pumped back out, while potassium does the reverse journey. The ions formed are negative,. Web a sodium ion is formed when a sodium atom loses a single electron. Lose electrons any astronomical body that revolves around a larger body is called a.
PPT Valence Electrons PowerPoint Presentation ID651377
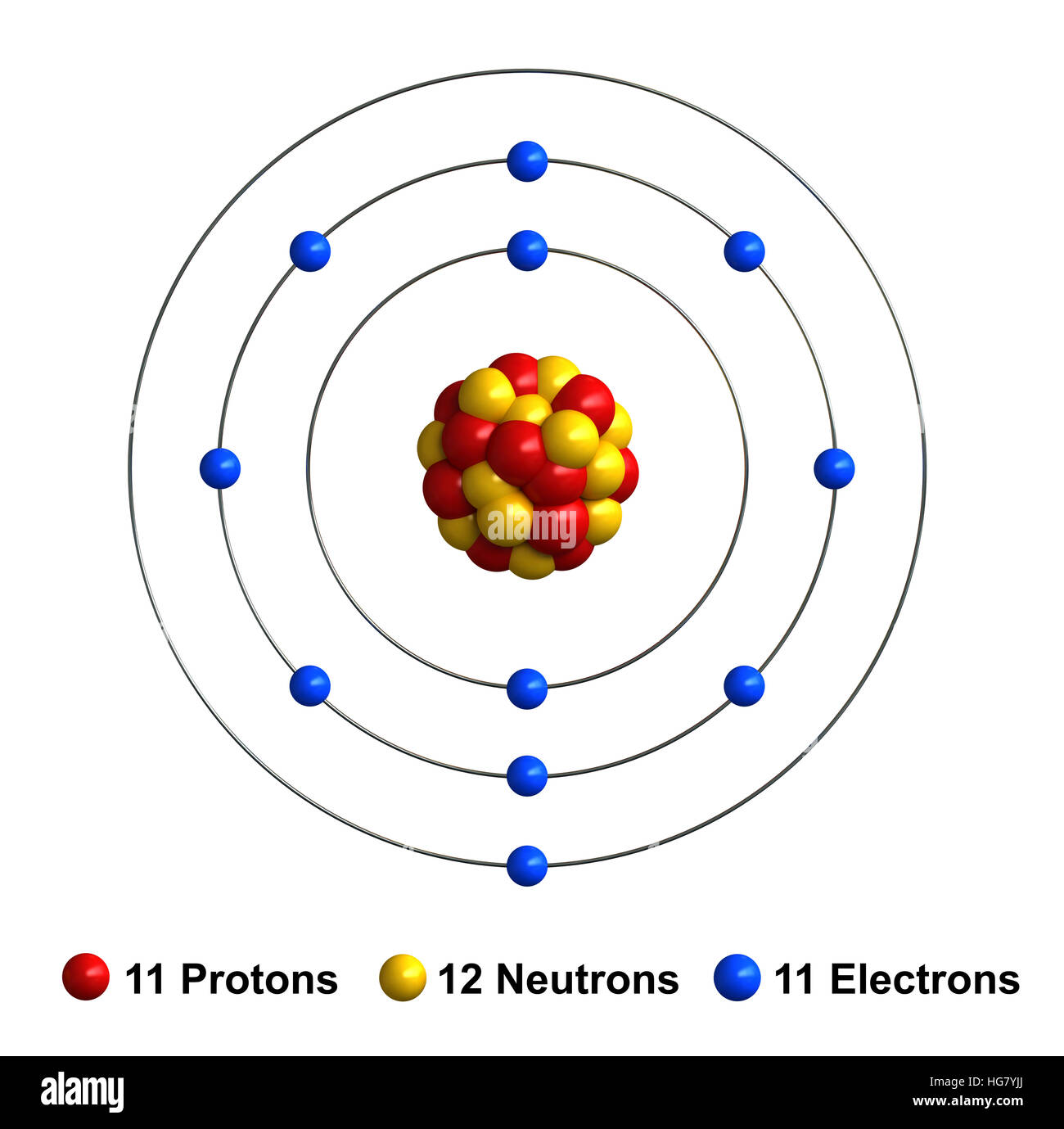
Web sodium ions are used to build up electrical gradients in the firing of neurons in the brain. Web when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Web sodium is a chemical element with the symbol na (from latin natrium) and atomic number 11. The term isoelectronic refers.
Sodium Atom Science Notes and Projects
This involves sodium (and its big brother potassium) diffusing through cell membranes. Sodium diffuses in and is pumped back out, while potassium does the reverse journey. Web sodium ions are used to build up electrical gradients in the firing of neurons in the brain. Web when sodium atoms form sodium ions, they a. Thus, if you commit the information in.
化学键原子如何结合?把原子结合在一起的力是什么?——Owlcation 188宝金博官网到底是哪个
Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. (in chapter 9 “chemical bonds”, we will discuss why atoms form the charges they do.) Sodium diffuses in and is pumped back out, while potassium does the reverse journey. Web when sodium atoms form ions, they always form.
3d render of atom structure of sodium isolated over white background
Web when sodium atoms form sodium ions, they a. Lose electrons any astronomical body that revolves around a larger body is called a. Sodium diffuses in and is pumped back out, while potassium does the reverse journey. Its only stable isotope is 23 na. The ions formed are negative,.
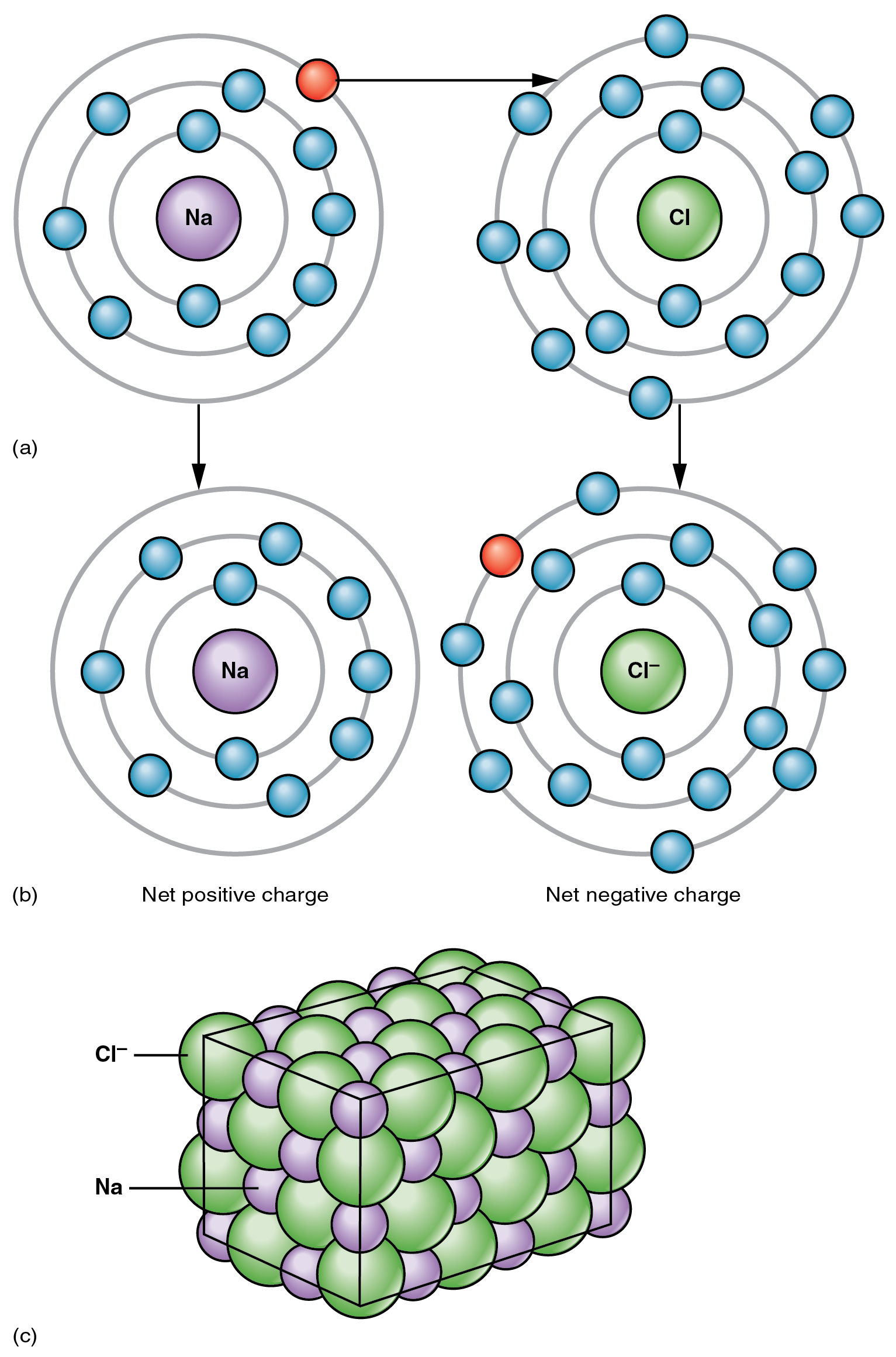
3.2 Ions The Basics of General, Organic, and Biological Chemistry
Thus, if you commit the information in table 3.2 to memory, you will always know what charges most atoms form. In 1807 sir humphry davy became the first to prepare sodium in its elemental form, applying electrolysis to fused sodium hydroxide (naoh). (in chapter 9 “chemical bonds”, we will discuss why atoms form the charges they do.) It is now.
Atom sodium Royalty Free Vector Image VectorStock
Thus, if you commit the information in table 3.2 to memory, you will always know what charges most atoms form. The ion is electrically charged, while the atom is electrically neutral. The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. Web the sodium ion, na.
How reactive is an atom of Sodium(Na) and why?
Lose electrons any astronomical body that revolves around a larger body is called a. Web the sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Web sodium is a chemical element with the symbol na (from latin natrium) and atomic number 11. Web properties and production sir humphry davy because.
Formation of Ions and Ionic Compounds Good Science
Lose electrons any astronomical body that revolves around a larger body is called a. Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. Sodium is an alkali metal, being in group 1 of the periodic table. The ions formed are negative,. It is now the same as.
The top panel of this figure shows the orbit model of a sodium atom and
It is now the same as that of the noble gas neon. Web the sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level. Web when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. In 1807 sir humphry davy became.
Ionic Properties
The ions formed are negative,. Web when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Web when sodium atoms form sodium ions, they a. Web when sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. This involves sodium (and.
Web When Sodium Atoms Form Ions, They Always Form A 1+ Charge, Never A 2+ Or 3+ Or Even 1− Charge.
Web when sodium atoms form sodium ions, they a. Web sodium ions are used to build up electrical gradients in the firing of neurons in the brain. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23 na.
Web When Sodium Atoms Form Ions, They Always Form A 1+ Charge, Never A 2+ Or 3+ Or Even 1− Charge.
Web sodium is a chemical element with the symbol na (from latin natrium) and atomic number 11. This involves sodium (and its big brother potassium) diffusing through cell membranes. Compare and contrast a sodium atom and a sodium ion and. Web a sodium ion is formed when a sodium atom loses a single electron.
Web When Sodium Atoms Form Sodium Ions, They A.
Sodium diffuses in and is pumped back out, while potassium does the reverse journey. The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. The ions formed are negative,. Web the sodium ion, na +, has the electron configuration with an octet of electrons from the second principal energy level.
Thus, If You Commit The Information In Table \(\Pageindex{1}\) To Memory, You Will Always Know What Charges Most Atoms Form.
Thus, if you commit the information in table 3.2 to memory, you will always know what charges most atoms form. (in chapter 9 “chemical bonds”, we will discuss why atoms form the charges they do.) Web properties and production sir humphry davy because sodium is extremely reactive, it never occurs in the free state in earth’s crust. The ion is electrically charged, while the atom is electrically neutral.