What Type Of Elements Form Covalent Bonds
What Type Of Elements Form Covalent Bonds - Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. In general, they are nonmetals with similar electronegativities. Web molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (h 2, n 2, cl 2, h 2 o, nh 3) together with all organic compounds. Web diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. Web ionic and covalent bonds introduction. It is a type of chemical. They are located toward the center of the periodic table, according to howstuffworks. Web there are two basic types of covalent bonds: In structural representations of molecules, covalent bonds are indicated by solid lines connecting pairs of atoms;
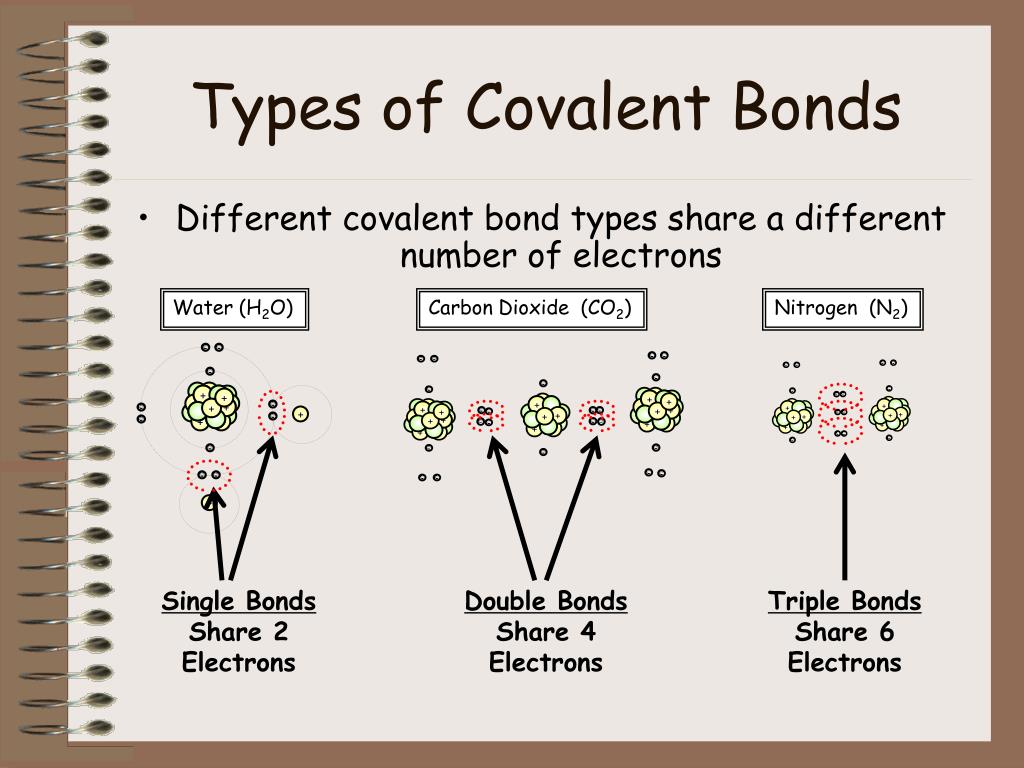
For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. Web there are actually three different types of chemical bonds, called covalent, ionic, and metallic bonds. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. This type of covalent bond exists where the unequal sharing of electrons occurs due to the. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. This type of covalent bond is. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. Covalent bonding is the sharing of electrons between atoms.
Web double bonds triple bond. Ionic bonding is the complete transfer of valence electron (s) between atoms. Web molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (h 2, n 2, cl 2, h 2 o, nh 3) together with all organic compounds. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. Web there are two basic types of covalent bonds: Containing covalent bonds between two of the same type of atom are only a few examples of the vast number of molecules that can form. This type of bonding occurs between two. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. A covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons.
Covalent Bonds Biology for NonMajors I
Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Figure 7.4 illustrates why this bond is formed. A covalent bond is the force of attraction that holds together two.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
This type of covalent bond is. Web there are actually three different types of chemical bonds, called covalent, ionic, and metallic bonds. Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. It is a type of chemical. Web double bonds triple bond.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Each type of bond is described below. Web double bonds triple bond. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. They are located toward the center of the periodic table, according to howstuffworks. Two different atoms can also.
Covalent Bonds The Basics of Chemical Bonding
Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. This type of covalent bond is. Covalent bonding is the sharing of electrons between atoms. Web there are two basic types of covalent bonds: A covalent bond is the force of attraction that holds together two.
Explain what a covalent bond is, what types of elements form covalent
Containing covalent bonds between two of the same type of atom are only a few examples of the vast number of molecules that can form. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Ionic bonding is the complete transfer of valence electron (s) between atoms. Web the chemical elements most likely to form covalent bonds are those.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Web diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. This type of bonding occurs between two. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. Each type of bond is described below. This type of covalent bond exists where the.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (h 2, n 2, cl 2, h 2 o, nh 3) together with all organic compounds. Web the sharing of electrons between atoms is called a covalent bond,.
Forms of Binding in Crystals Overall Science
They are located toward the center of the periodic table, according to howstuffworks. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. Each type of bond is described below. Ionic bonding is the complete transfer of valence electron (s).
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. In structural representations of molecules, covalent bonds are indicated by solid lines connecting pairs of atoms; Web the chemical elements most likely to form covalent bonds are those that share.
Covalent Bond Biology Dictionary
Ionic bonding is the complete transfer of valence electron (s) between atoms. This type of covalent bond exists where the unequal sharing of electrons occurs due to the. Figure 7.4 illustrates why this bond is formed. Web molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (h 2, n 2, cl 2, h 2.
Web The Sharing Of Electrons Between Atoms Is Called A Covalent Bond, And The Two Electrons That Join Atoms In A Covalent Bond Are Called A Bonding Pair Of Electrons.
Each type of bond is described below. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. In general, they are nonmetals with similar electronegativities. Web double bonds triple bond.
Web Nonmetal Atoms Frequently Form Covalent Bonds With Other Nonmetal Atoms.
Ionic bonding is the complete transfer of valence electron (s) between atoms. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another element to form an ionic bond. It is a type of chemical. A triple bond is formed when three pairs of electrons are shared between the two participating atoms.
Web There Are Two Basic Types Of Covalent Bonds:
Web molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (h 2, n 2, cl 2, h 2 o, nh 3) together with all organic compounds. This type of covalent bond is. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom than the other. In structural representations of molecules, covalent bonds are indicated by solid lines connecting pairs of atoms;
Covalent Bonding Is The Sharing Of Electrons Between Atoms.
Web ionic and covalent bonds introduction. Two different atoms can also share electrons and form covalent bonds. Figure 7.4 illustrates why this bond is formed. They are located toward the center of the periodic table, according to howstuffworks.