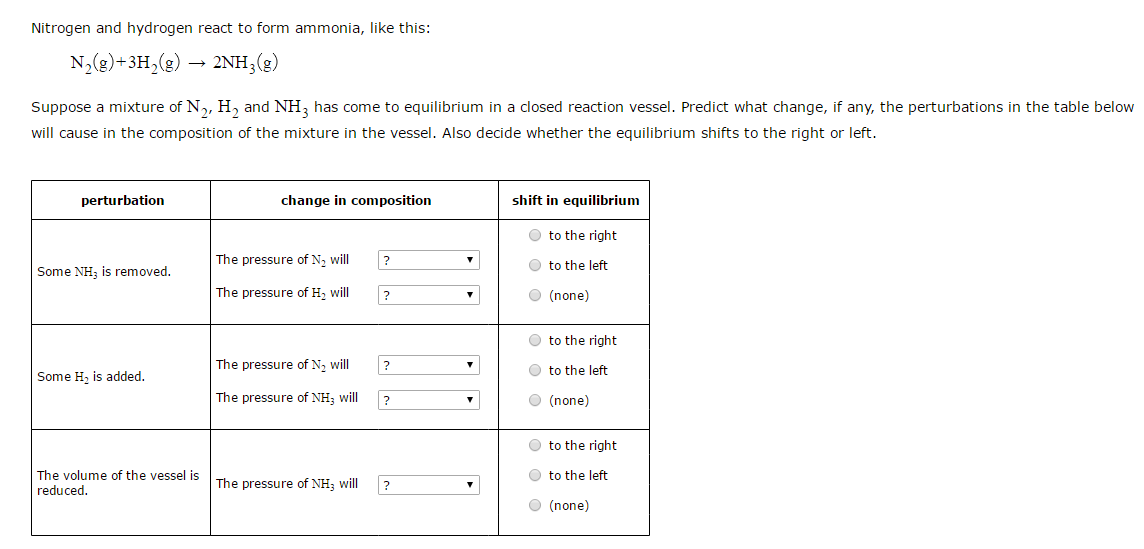
Reaction Of Hydrogen And Nitrogen To Form Ammonia
Reaction Of Hydrogen And Nitrogen To Form Ammonia - The volume of nitrogen (n₂) gas = 7.7 ml. Thus, 7.7 ml = 0.0077 l. Asked • 12/18/20 hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. The reaction between ammonia and hydrogen to give ammonia is known as haber process. N₂+ h₂→nh₃ to balance ,follow the following steps. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Hydrogen gas, h 2, reacts with nitrogen gas, n 2, to form ammonia gas, nh 3, according to the equation. If 4.0 moles of h2 with 2.0 moles of n2. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? 3h2 + n2 → 2nh3 according to the stoichiometric equation, 6g of h2 combines with 28g of n2.
Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst. Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. The volume of nitrogen (n₂) gas = 7.7 ml. Web reaction of hydrogen and nitrogen to form ammonia. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. The haber process is also known as the. Asked • 12/18/20 hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Web hydrogen gas and nitrogen gas react to form ammonia gas. Web complete step by step answer: The reaction between ammonia and hydrogen to give ammonia is known as haber process.
In the reaction, bonds between two atoms of nitrogen molecule and atoms of hydrogen molecules are broken and new bonds for ammonia are formed. Web hydrogen gas and nitrogen gas react to form ammonia gas. It is the simplest stable compound of these elements and serves as a starting. If 4.0 moles of h2 with 2.0 moles of n2. 1 ml = 0.001 l. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? I mean to say that, when hydrogen (g) and nitrogen (g) combines together. Web in the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of nitrogen? So, 1.09g of h2 will combine. Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst.
Solved Nitrogen and hydrogen react to form ammonia, like
I mean to say that, when hydrogen (g) and nitrogen (g) combines together. In the reaction, bonds between two atoms of nitrogen molecule and atoms of hydrogen molecules are broken and new bonds for ammonia are formed. The reaction between ammonia and hydrogen to give ammonia is known as haber process. Web the following chemical reaction takes place when hydrogen.
Solved Nitrogen and hydrogen react to form ammonia, like
So, 1.09g of h2 will combine. The haber process is also known as the. The volume of nitrogen (n₂) gas = 7.7 ml. Web ammonia (nh3), colourless, pungent gas composed of nitrogen and hydrogen. Web a combination reaction is a reaction in which two or more reactants combine to form a single product.
Solved 1. Nitrogen and hydrogen react to form ammonia N2(g)
In the reaction, bonds between two atoms of nitrogen molecule and atoms of hydrogen molecules are broken and new bonds for ammonia are formed. Web in the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of nitrogen? Simonndavis03 simonndavis03 01/04/2019 chemistry middle.
Solved Nitrogen and hydrogen react to form ammonia, like
1 ml = 0.001 l. Asked • 12/18/20 hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 —> 2 nh3. Web nitrogen reacts with hydrogen to give ammonia. The haber process is also known as the. Web nitrogen and hydrogen combine to form ammonia via the following reaction:
Solved Nitrogen And Hydrogen Combine To Form Ammonia In T...
Hydrogen gas, h 2, reacts with nitrogen gas, n 2, to form ammonia gas, nh 3, according to the equation. 1 ml = 0.001 l. Web nitrogen reacts with hydrogen to give ammonia. Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. Web nitrogen and hydrogen combine to form ammonia via the following reaction:
Solved Hydrogen reacts with nitrogen to form ammonia (NH_3)
1 ml = 0.001 l. 3h2 + n2 → 2nh3 according to the stoichiometric equation, 6g of h2 combines with 28g of n2. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. I mean to say that, when hydrogen (g) and nitrogen (g) combines together. The reaction.
Solved Nitrogen and hydrogen react to produce ammonia
3h2 + n2 → 2nh3 according to the stoichiometric equation, 6g of h2 combines with 28g of n2. Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. Simonndavis03 simonndavis03 01/04/2019 chemistry middle school. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. It is.
[Best Answer] hydrogen gas combines with nitrogen to form Ammonia
Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. So if each coefficient is multiplied by a mole, the balanced. So, 1.09g of h2 will combine. Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions,.
Solved Nitrogen and hydrogen react to form ammonia, like
So if each coefficient is multiplied by a mole, the balanced. Web in the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of nitrogen? Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. Web complete step by step answer:.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Web ammonia (nh3), colourless, pungent gas composed of nitrogen and hydrogen. Web complete step by step answer: Calculate the volume of the ammonia gas formed when nitrogen reacts with \\[6\\,litres\\] of hydrogen. So, 1.09g of h2 will combine. N₂+ h₂→nh₃ to balance ,follow the following steps.
3H2 + N2 → 2Nh3 According To The Stoichiometric Equation, 6G Of H2 Combines With 28G Of N2.
Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; In the reaction, bonds between two atoms of nitrogen molecule and atoms of hydrogen molecules are broken and new bonds for ammonia are formed. Hydrogen gas, h 2, reacts with nitrogen gas, n 2, to form ammonia gas, nh 3, according to the equation. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the.
Reaction Of Hydrogen And Nitrogen To Form Ammonia Hydrogen Gas, H2, Reacts With Nitrogen Gas, N2, To Form Ammonia Gas, Nh3, According To The Equation 3H2.
Web nitrgen reacts with hydrogen to form ammonia.this is an exothermic reaction. Web hydrogen gas and nitrogen gas react to form ammonia gas. Simonndavis03 simonndavis03 01/04/2019 chemistry middle school. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g).
Web In The Reaction Of Nitrogen Gas, N2, With Hydrogen Gas, H2, To Form Ammonia Gas, Nh3, How Many Moles Of Hydrogen Are Needed To React With Two Moles Of Nitrogen?
Web we see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2 molecules of ammonia. Calculate the volume of the ammonia gas formed when nitrogen reacts with \\[6\\,litres\\] of hydrogen. Web nitrogen and hydrogen combine to form ammonia via the following reaction: 1 ml = 0.001 l.
Web Answer (1 Of 3):
Thus, 7.7 ml = 0.0077 l. Web nitrogen reacts with hydrogen to give ammonia. So if each coefficient is multiplied by a mole, the balanced. I mean to say that, when hydrogen (g) and nitrogen (g) combines together.
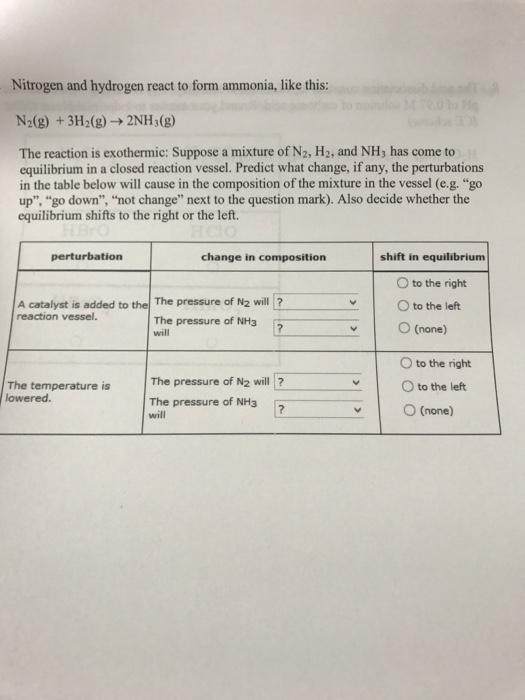
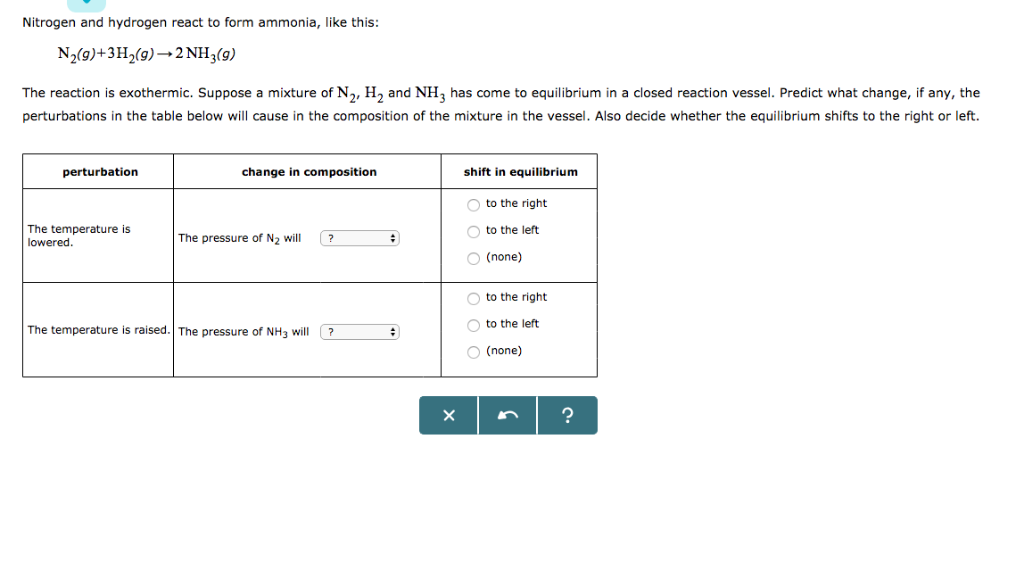
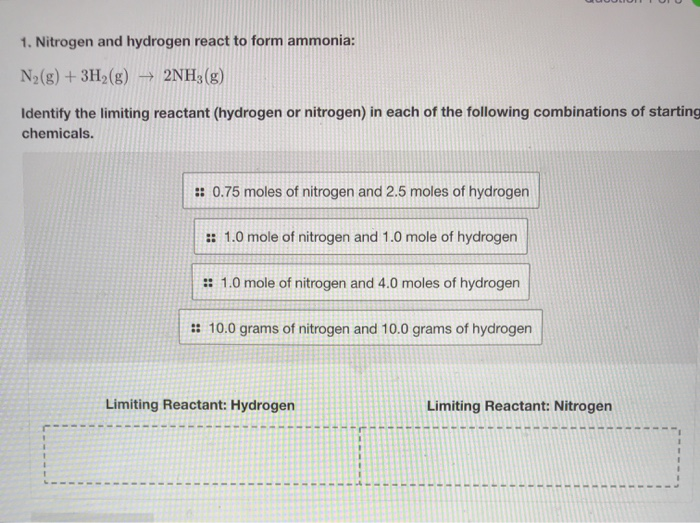



![[Best Answer] hydrogen gas combines with nitrogen to form Ammonia](https://hi-static.z-dn.net/files/dc1/9e1c135271e8d11c1d9621fa70f7ea35.jpg)