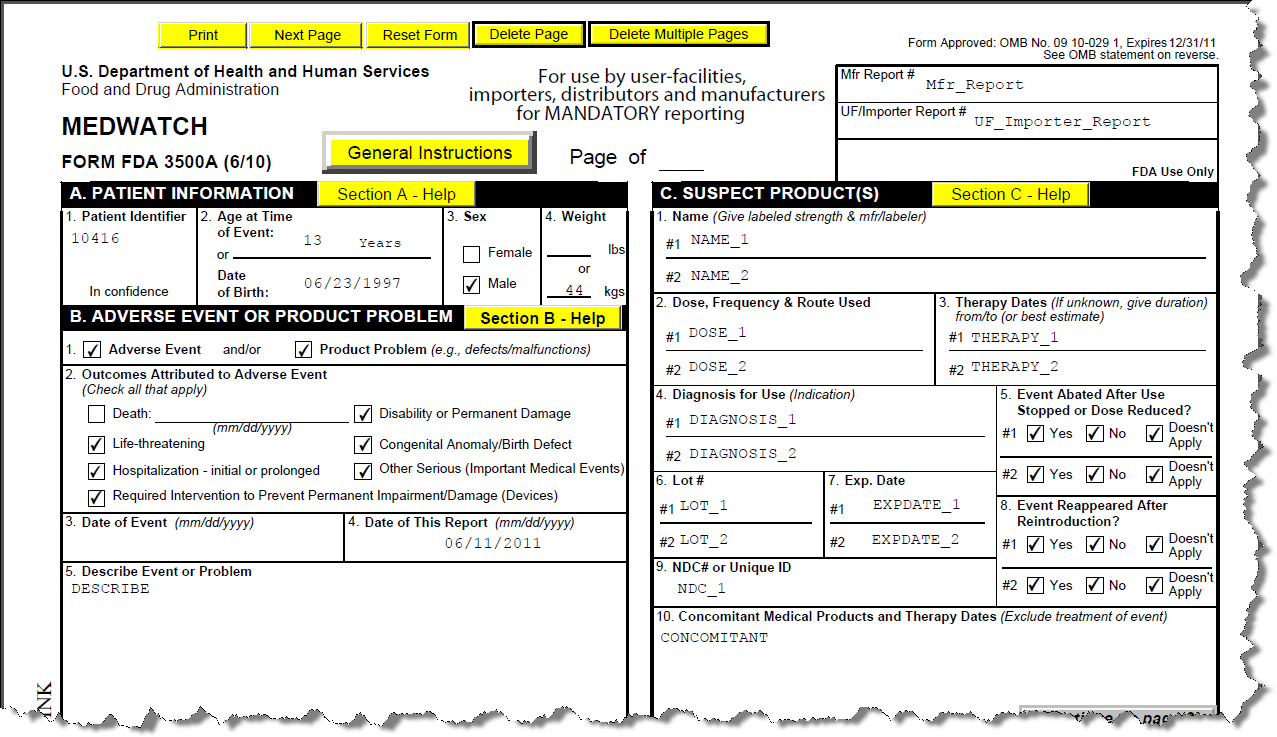
Med Watch Form
Med Watch Form - Safety alerts for human medical products (drugs, biologics. Easily fill out pdf blank, edit, and sign them. Web complete medwatch form online with us legal forms. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Patient or patient healthcare representative: Web a copy of a dhmh medwatch form and instructions are available at the links below. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical. Save or instantly send your ready documents. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web online using the medwatch online reporting form;
Web complete medwatch form online with us legal forms. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Save or instantly send your ready documents. Safety alerts for human medical products (drugs, biologics. Web online using the medwatch online reporting form; Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Patient or patient healthcare representative: Web form fda 3500 author: Easily fill out pdf blank, edit, and sign them. For voluntary reporting of adverse events, product problems and product use/medication errors created date:
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Safety alerts for human medical products (drugs, biologics. Save or instantly send your ready documents. Web online using the medwatch online reporting form; Web complete medwatch form online with us legal forms. Web form fda 3500 author: Easily fill out pdf blank, edit, and sign them. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. For voluntary reporting of adverse events, product problems and product use/medication errors created date:
FDA MedWatch Pioglitazonecontaining Medicines Drug Safety
Web form fda 3500 author: Web a copy of a dhmh medwatch form and instructions are available at the links below. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Safety alerts for human medical products (drugs, biologics. For voluntary reporting of adverse events, product problems and product use/medication errors created date:
Regulatory Submissions Product Documentation
For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Easily fill out pdf blank, edit, and sign them. Web online using the medwatch online reporting form; Web form.
Medwatch Instructions For Medwatch Form 3500 Voluntary Reporting Of
Patient or patient healthcare representative: Easily fill out pdf blank, edit, and sign them. Web a copy of a dhmh medwatch form and instructions are available at the links below. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web form fda 3500 author:
fda form 3500a Fill out & sign online DocHub
Web form fda 3500 author: Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web reporting can be done through our online reporting portal or by downloading,.
MedWatch Forms YouTube
Web online using the medwatch online reporting form; Web a copy of a dhmh medwatch form and instructions are available at the links below. Easily fill out pdf blank, edit, and sign them. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web reporting can be done through our online reporting portal or by.
MedWatchLogo1 KnowYourOTCS
For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web a copy of a dhmh medwatch form and instructions are available at the links below. Safety alerts for.
The Importance of HighQuality Reporting of an SAE Case Report The
Easily fill out pdf blank, edit, and sign them. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web online using the medwatch online reporting form; Safety alerts for human medical products (drugs, biologics. Web reporting can be done through our online.
Form FDA 3500B MEDWATCH Consumer Voluntary Reporting Free Download
Easily fill out pdf blank, edit, and sign them. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web medwatch is the food and drug administration's (fda).
3 Medwatch Form Templates free to download in PDF
Safety alerts for human medical products (drugs, biologics. Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Web form fda 3500 author: Patient or patient healthcare representative: Easily fill out pdf blank, edit, and sign them.
Fda Medwatch Form Fill Out and Sign Printable PDF Template signNow
Easily fill out pdf blank, edit, and sign them. Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. For voluntary reporting of adverse events, product problems and.
Web A Copy Of A Dhmh Medwatch Form And Instructions Are Available At The Links Below.
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Save or instantly send your ready documents. Web form fda 3500 author: Web complete medwatch form online with us legal forms.
Easily Fill Out Pdf Blank, Edit, And Sign Them.
For voluntary reporting of adverse events, product problems and product use/medication errors created date: Web online using the medwatch online reporting form; Medwatch form (required for all “brand medically necessary” prescriptions) instructions for completing the medwatch form. Safety alerts for human medical products (drugs, biologics.
Patient Or Patient Healthcare Representative:
Web reporting can be done through our online reporting portal or by downloading, completing and then submitting fda form 3500 (health professional) or 3500b (consumer/patient) to medwatch: Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical.