Lithium Electron Configuration Long Form
Lithium Electron Configuration Long Form - Electron configuration of lithium is [he] 2s1. A vertical column in the periodic table. 1s 2 2s 2 2p 1: Web electron configurations describe where electrons are located around the nucleus of an atom. The valence shell of lithium is the second shell, which contains one electron in the 2s subshell. Possible oxidation states are +1. Electron configuration of beryllium (be) [he] 2s 2: Web in any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Lithium is the third element with a total of 3 electrons. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell.
From its position, we know that it has 1 valence electron in the 2s orbital series (because it's in the second period): Li readily oxidizes to lithium oxide on exposure to air. Electron configuration of lithium is [he] 2s1. Web the electronic configuration of lithium is 1s² 2s¹. The complete electron configuration of lithium is 1s22s1.lithium has 1 valence electrons around the nucleus and the atomic number is 3. Your starting point here will be the electron configuration of a neutral lithium atom, li. Web in any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Possible oxidation states are +1. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 lithium: A horizontal row in the periodic table.
Electron configuration of beryllium (be) [he] 2s 2: For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. It is the lightest among metals and has a high specific heat capacity. Members of a group typically have similar properties and electron configurations in their outer shell. Electron configuration of lithium is [he] 2s1. From its position, we know that it has 1 valence electron in the 2s orbital series (because it's in the second period): Lithium reacts with water easily, but with noticeably less vigor than other alkali metals. Web electron configurations describe where electrons are located around the nucleus of an atom. An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The chemical symbol for lithium is li.
Lithium Table of Elements by Shrenil Sharma
Web electron configuration of lithium is [he] 2s1. We’ll also look at why lithium forms a 1+ ion and how the electron configuration for li+. Web the electronic configuration of lithium is 1s² 2s¹. The helium atom contains two protons and two electrons. The chemical symbol for lithium is li.
Electron Configuration Of Carbon And Full Orbital Diagram
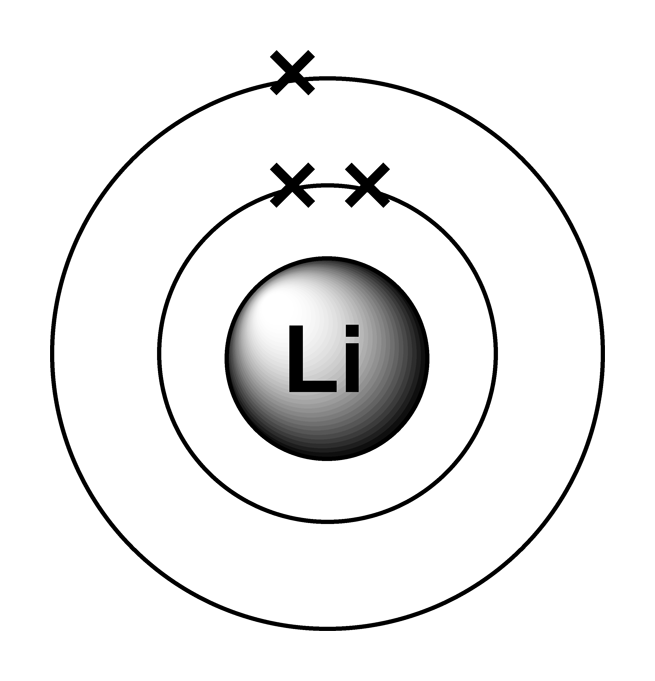
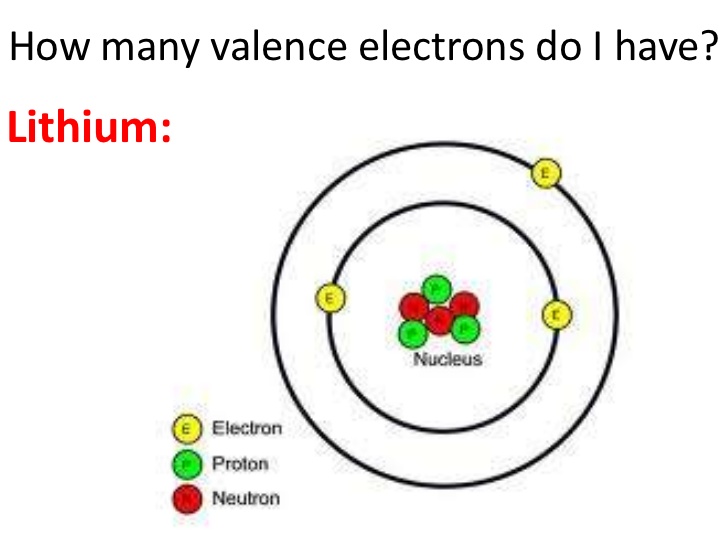
This means that lithium has three electrons in total, with two electrons occupying the innermost shell (1s subshell) and one electron in the second shell (2s subshell). The shell diagram for a lithium atom (figure \(\pageindex{1}\)). Since 1s can only hold two electrons the remaining electron for. The helium atom contains two protons and two electrons. For each atom the.
Lithium Element With Reactions, Properties, Uses, & Price Periodic Table
Web thus, the electron configuration and orbital diagram of lithium are: 1 depicts how these two trends in. Web how to write the electron configuration for lithium. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. We’ll also look at why lithium forms a 1+.
FicheiroElectron shell 003 Lithium.svg Wikipédia, a enciclopédia
Web electron configurations describe where electrons are located around the nucleus of an atom. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Web thus, the electron configuration and orbital diagram of lithium are: For example, the electron configuration of lithium, 1s²2s¹, tells us.
Lithium Electron Configuration YouTube
An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 lithium: Web in any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the.
Lithium's Atomic Structure YouTube
Lithium is the third element with a total of 3 electrons. The shell diagram for a lithium atom (figure \(\pageindex{1}\)). Electron configuration of helium (he) 1s 2: 1s 2 2s 2 2p 1: Web answer (1 of 4):
How Do We Can Find A Lithium Electron Configuration (Li)
Possible oxidation states are +1. Web how to write the electron configuration for lithium. Electron configuration of lithium (li) [he] 2s 1: The valence shell of lithium is the second shell, which contains one electron in the 2s subshell. The shell diagram for a lithium atom (figure \(\pageindex{1}\)).
What Is Electron Configuration?
_↑ ↓ _ _↑__ 1s 2 2s 1 Lithium reacts with water easily, but with noticeably less vigor than other alkali metals. Web electron configuration of hydrogen (h) 1s 1: The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 )..
Lithium electronic configuration,how to Write lithium electronic
Possible oxidation states are +1. A vertical column in the periodic table. Web electron configurations of the elements (data page) this page shows the electron configurations of the neutral gaseous atoms in their ground states. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms.
Lithium Atom Orbital Diagram / Hydrogen Like Atoms Lithium (recall
For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. The valence shell of lithium is the second shell, which contains one electron in the 2s subshell. Web like the other alkali metals (which are sodium (na), potassium (k), rubidium (rb), caesium (cs), and.
Lithium Is Flammable, And It Is Potentially Explosive When Exposed To Air And Especially To Water, Though Less So Than The Other Alkali Metals.
Web electron configurations describe where electrons are located around the nucleus of an atom. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell (2s) has 1 electron. _↑ ↓ _ _↑__ 1s 2 2s 1 17, 2023, 9:19 pm et (ap) show more lithium lithium (li), chemical element of group 1 (ia) in the periodic table, the alkali metal group, lightest of the solid elements.
Web The Electron Configuration And The Orbital Diagram Are:
Lithium is the third element with a total of 3 electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ). Web the electron configuration of lithium (li) is 1s2 2s1. Li is a silvery white metal.
Web The Electronic Configuration Of Lithium Is 1S² 2S¹.
Web electron configuration of lithium is [he] 2s1. Web thus, the electron configuration and orbital diagram of lithium are: For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. A quick look in the periodic table will reveal that lithium is located in period 2, group 1, and that it has an atomic number equal to 3.
Possible Oxidation States Are +1.
Web how to write the electron configuration for lithium. Since 1s can only hold two electrons the remaining electron for. This means that lithium has three electrons in total, with two electrons occupying the innermost shell (1s subshell) and one electron in the second shell (2s subshell). Its atomic weight is 6.941 u.





/lithiumatom-56a12c335f9b58b7d0bcc103.jpg)

