Iso 14971 Template
Iso 14971 Template - Risk management plan risk acceptability criteria residual risk. Web templates iso 14971 templates updated june 20, 2023 template: Web risk per iso 14971 is defined as the combination of the probability of occurrence of harm and the severity of that harm. Web iso 14971:2019(e) introduction the requirements contained in this document provide manufacturers with a framework within which experience, insight and judgment are. Web this document provides guidance on the development, implementation and maintenance of a risk management system for medical devices according to iso 14971:2019. Web iso 14971:2019 is the international standard for risk management in medical devices. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Manufacturers can use the standard to identify and. Here are all our posts on this standard, and also all questions our consulting clients have asked us about. Web iso 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (ivd) medical devices, to estimate and.
Oliver eidel template download this is a free template,. Web iso 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (ivd) medical devices, to estimate and. Web templates iso 14971 templates updated june 20, 2023 template: Web iso 14971:2019 (en), medical devices — application of risk management to medical devices follow en fr es foreword iso (the international organization for standardization) is a. Iso 14971:2019 has been recognized as the consensus standard by the fda. Web iso 14971:2019 is the international standard for risk management in medical devices. Web word template for avery 3671 multipurpose labels, 64 x 45 mm, 18 per sheet. Web word template for avery l7971 freezer labels, 38,1 x 21,2 mm, 5 per sheet. Web iso 14971 is the standard for risk management of medical device software. Web iso 14971:2019 is an international standard that guides the application of risk management to medical devices.
Web iso 14971 is the standard for risk management of medical device software. Web iso 14971:2019 (en), medical devices — application of risk management to medical devices follow en fr es foreword iso (the international organization for standardization) is a. Oliver eidel iso 14971 is the standard for risk management of medical. Web word template for avery 3671 multipurpose labels, 64 x 45 mm, 18 per sheet. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro diagnostic. Manufacturers can use the standard to identify and. Iso 14971:2019 has been recognized as the consensus standard by the fda. Web iso 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (ivd) medical devices, to estimate and. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Here are all our posts on this standard, and also all questions our consulting clients have asked us about.
ISO 149712019 Changes in the Current Version of ISO 14971 Oriel
The intent behind risk management. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (ivd) medical devices, to estimate and. Web.
Iso 14971 Risk Management Plan Example
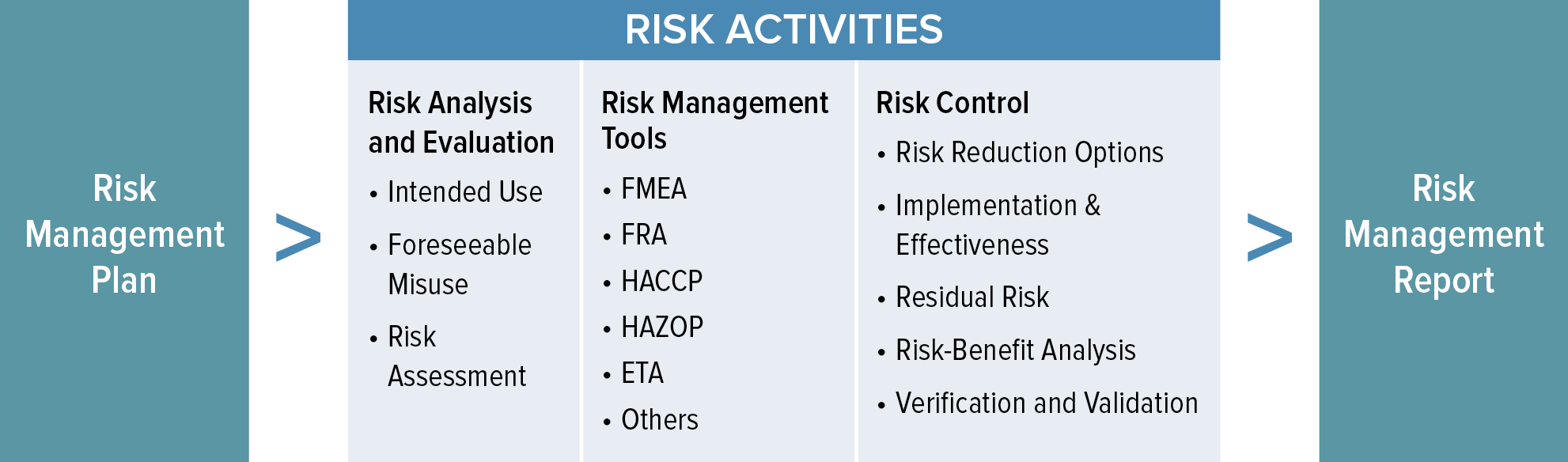
Web the 14971 wants you to analyze hazards, hazardous situations and harms, so that’s what you’ll find in the table :) here’s what happens: In the beginning, there’s a. Web iso 14971:2019 is an international standard that guides the application of risk management to medical devices. Web templates iso 14971 templates updated june 20, 2023 template: Web word template for.
ISO 14971 Risk Management Forms
Iso 14971:2019 has been recognized as the consensus standard by the fda. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro diagnostic. Web templates iso 14971 templates updated june 20, 2023 template: Web iso 14971 specifies a process through which the manufacturer of a medical device.
Iso14971 Risk Management Template Iso 14971 Risk Management Plan
Iso 14971, the standard for the application of risk management to medical devices, does not provide specific templates for documentation. Oliver eidel template download this is a free template,. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Web the 14971 wants you to analyze hazards, hazardous situations and harms, so that’s what you’ll find.
Iso14971 Risk Management Template / The Definitive Guide to ISO 14971
Oliver eidel iso 14971 is the standard for risk management of medical. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Here are all our posts on this standard, and also all questions our consulting clients have asked us about. Web iso.
ISO 14971 Key Risk Analysis Terms and Relationships Download
Web iso 14971:2019 (en), medical devices — application of risk management to medical devices follow en fr es foreword iso (the international organization for standardization) is a. Web iso 14971:2019 is the international standard for risk management in medical devices. The intent behind risk management. Web iso 14971 specifies a process through which the manufacturer of a medical device can.
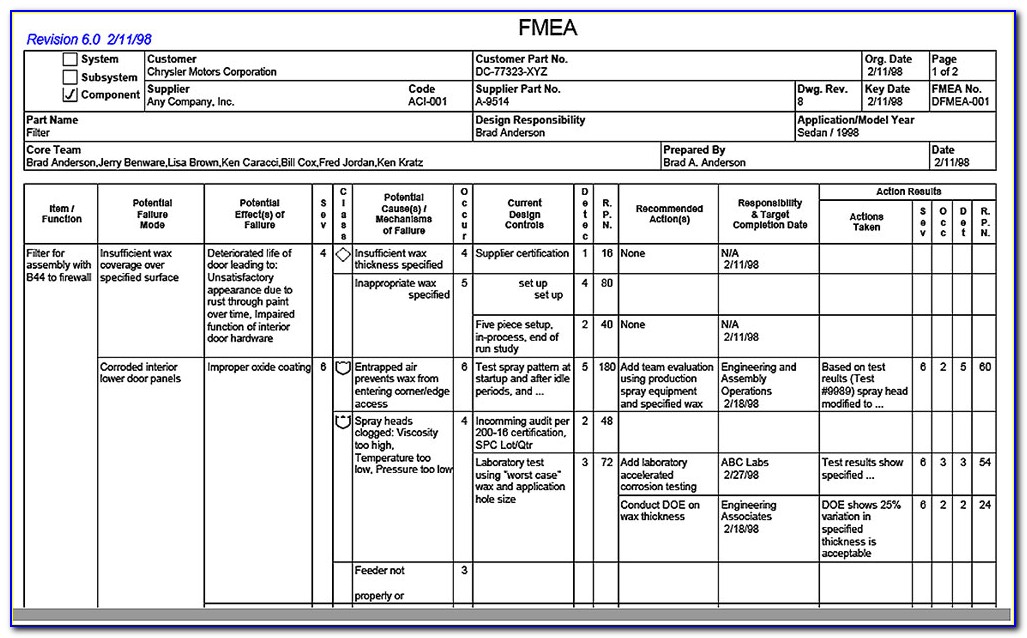
FMEA vs ISO 14971 Medical Device HQ
Web iso 14971:2019 is the international standard for risk management in medical devices. Risk management plan risk acceptability criteria residual risk. Web iso 14971:2019(e) introduction the requirements contained in this document provide manufacturers with a framework within which experience, insight and judgment are. Iso 14971, the standard for the application of risk management to medical devices, does not provide specific.
Why Use ISO 14971 vs. FMEA (Template Included)
Oliver eidel template download this is a free template,. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro diagnostic. Web iso 14971:2019 is an international standard that guides the application of risk management to medical devices. Web word template for avery 3671 multipurpose labels, 64 x.
Risk Management Plan Template (medical Device And Iso 14971)
Web word template for avery l7971 freezer labels, 38,1 x 21,2 mm, 5 per sheet. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971 templates templates updated june 8, 2023 iso 14971 templates dr. Web risk per iso 14971.
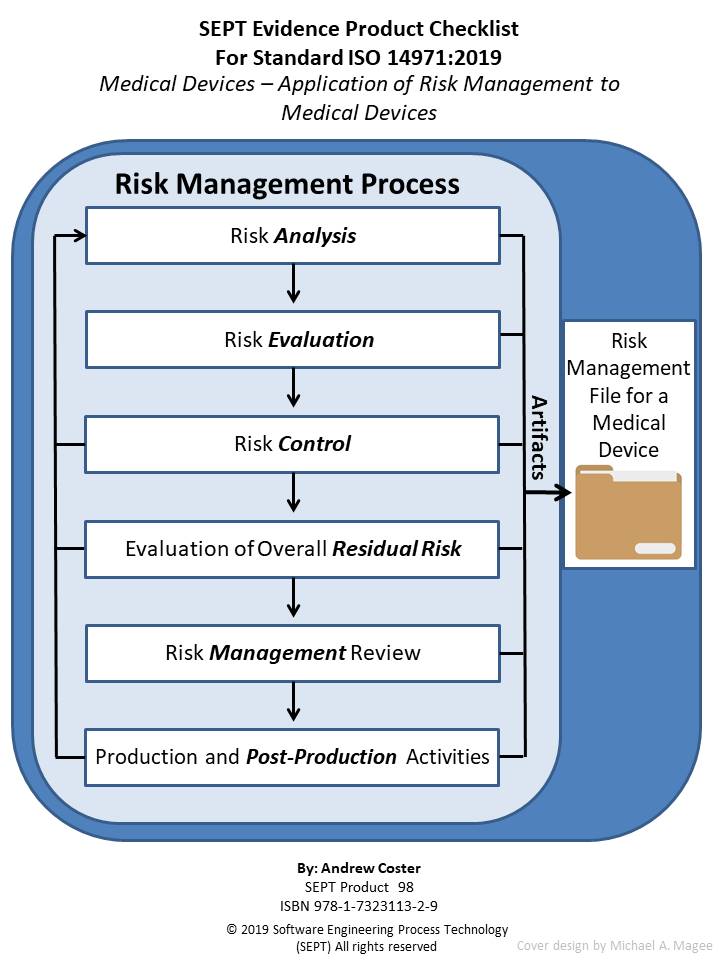
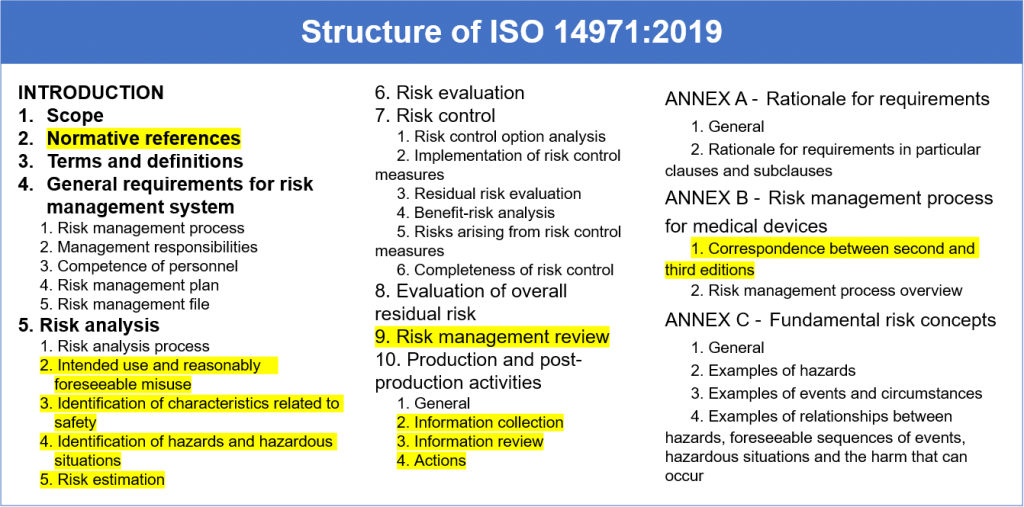
ISO 149712019 Medical devices Application of Risk Management to
Here are all our posts on this standard, and also all questions our consulting clients have asked us about. Oliver eidel iso 14971 is the standard for risk management of medical. Manufacturers can use the standard to identify and. Web key definitions implementing iso 14971 initiating risk management and design controls part 1: Web iso 14971:2019(e) introduction the requirements contained.
Iso 14971:2019 Has Been Recognized As The Consensus Standard By The Fda.
Web word template for avery 3671 multipurpose labels, 64 x 45 mm, 18 per sheet. Web iso 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (ivd) medical devices, to estimate and. Oliver eidel iso 14971 is the standard for risk management of medical. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in vitro diagnostic.
Web The 14971 Wants You To Analyze Hazards, Hazardous Situations And Harms, So That’s What You’ll Find In The Table :) Here’s What Happens:
The intent behind risk management. Web templates iso 14971 templates updated june 20, 2023 template: Web iso 14971:2019 is an international standard that guides the application of risk management to medical devices. Web this document provides guidance on the development, implementation and maintenance of a risk management system for medical devices according to iso 14971:2019.
In The Beginning, There’s A.
Web iso 14971 templates templates updated june 8, 2023 iso 14971 templates dr. Web iso 14971:2019(e) introduction the requirements contained in this document provide manufacturers with a framework within which experience, insight and judgment are. Oliver eidel template download this is a free template,. Web word template for avery l7971 freezer labels, 38,1 x 21,2 mm, 5 per sheet.
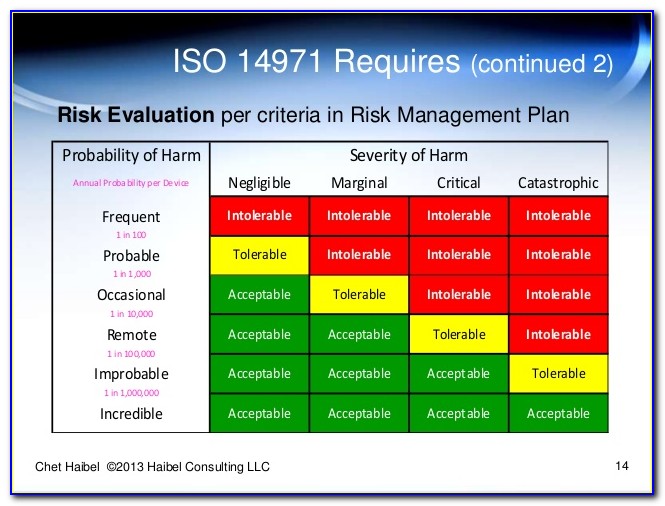
Risk Management Plan Risk Acceptability Criteria Residual Risk.
Web iso 14971:2019 (en), medical devices — application of risk management to medical devices follow en fr es foreword iso (the international organization for standardization) is a. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971:2019 is the international standard for risk management in medical devices. Web risk per iso 14971 is defined as the combination of the probability of occurrence of harm and the severity of that harm.


.png?width=3000&name=iso or fmea (1).png)