Iron Electron Configuration Long Form
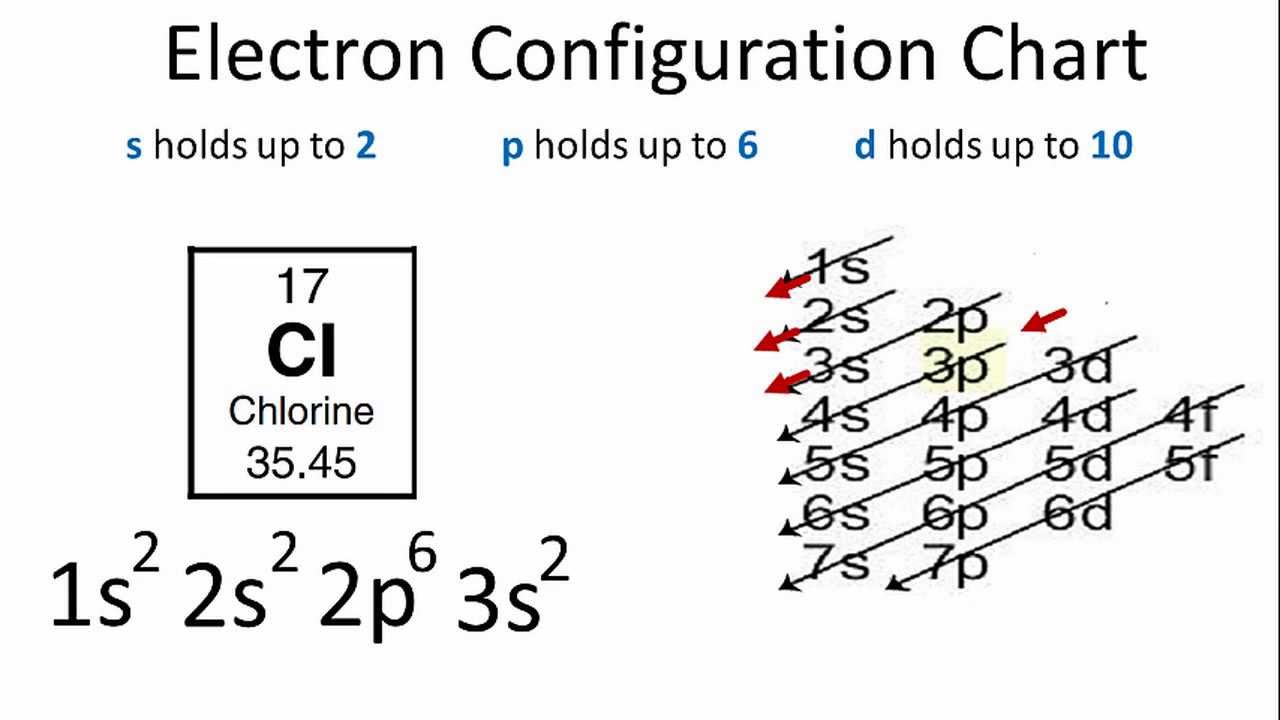
Iron Electron Configuration Long Form - The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and Web what we have is: Electron configuration through orbit (bohr principle) electron configuration through. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): Web the electronic iron configuration (e.g.) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6, and the form [ ar ] 4s2 3d6 is abbreviated. \[1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2} 3d^{6}\]. Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way. Iron is a member of group 8 and the first transition series of the periodic. Fe3+1s22s22p63s23p63d5 one other note on writing electron configurations:
[13] iron forms compounds mainly in the oxidation states +2. Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: The atomic number of iron is 26. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Web the average quantity of iron in the human body is about 4.5 grams (about 0.004 percent), of which approximately 65 percent is in the form of hemoglobin, which transports molecular oxygen from the lungs throughout the body; Offers the shortest lead times at the lowest variable cost, with flexibility to accommodate volume fluctuations and part. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Fe3+1s22s22p63s23p63d5 one other note on writing electron configurations: Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way. 1 percent in the various enzymes that control intracellular oxidation;
Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: Web 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 to write an electron configuration for any element you'll need to apply the aufbau principle and use a diagonal diagram. Electron configurations describe where electrons are located around the nucleus of an atom. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Nonetheless, it wasn’t easy to find the reason why it was written as [ ar ] 3d6 4s2 instead in some periodic table. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in. Electron configuration of neon (ne) [he] 2s 2 2p 6: Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way. We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom.
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration
Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Writing the electron configuration, you really only need the valence.
39+ Electron Configuration Of Iron Unabbreviated mariamirandag
Offers the shortest lead times at the lowest variable cost, with flexibility to accommodate volume fluctuations and part. Both are explained in the video below. This knowledge is essential for comprehending an element's chemical and physical characteristics, such as its reactivity, magnetic properties, spectral characteristics, and position in the periodic table. Web electron configuration the arrangements of electrons above the.
Electron Configurations Long Form YouTube
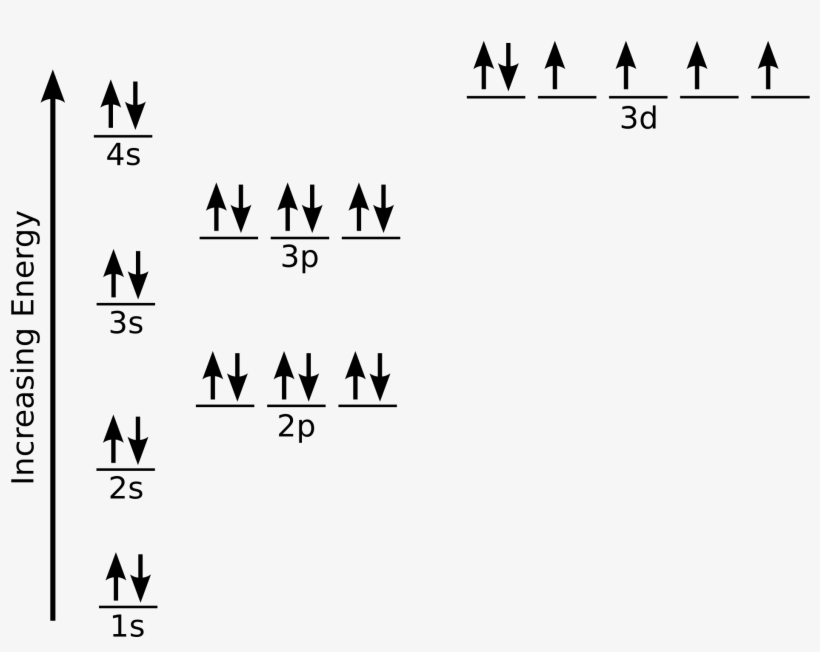
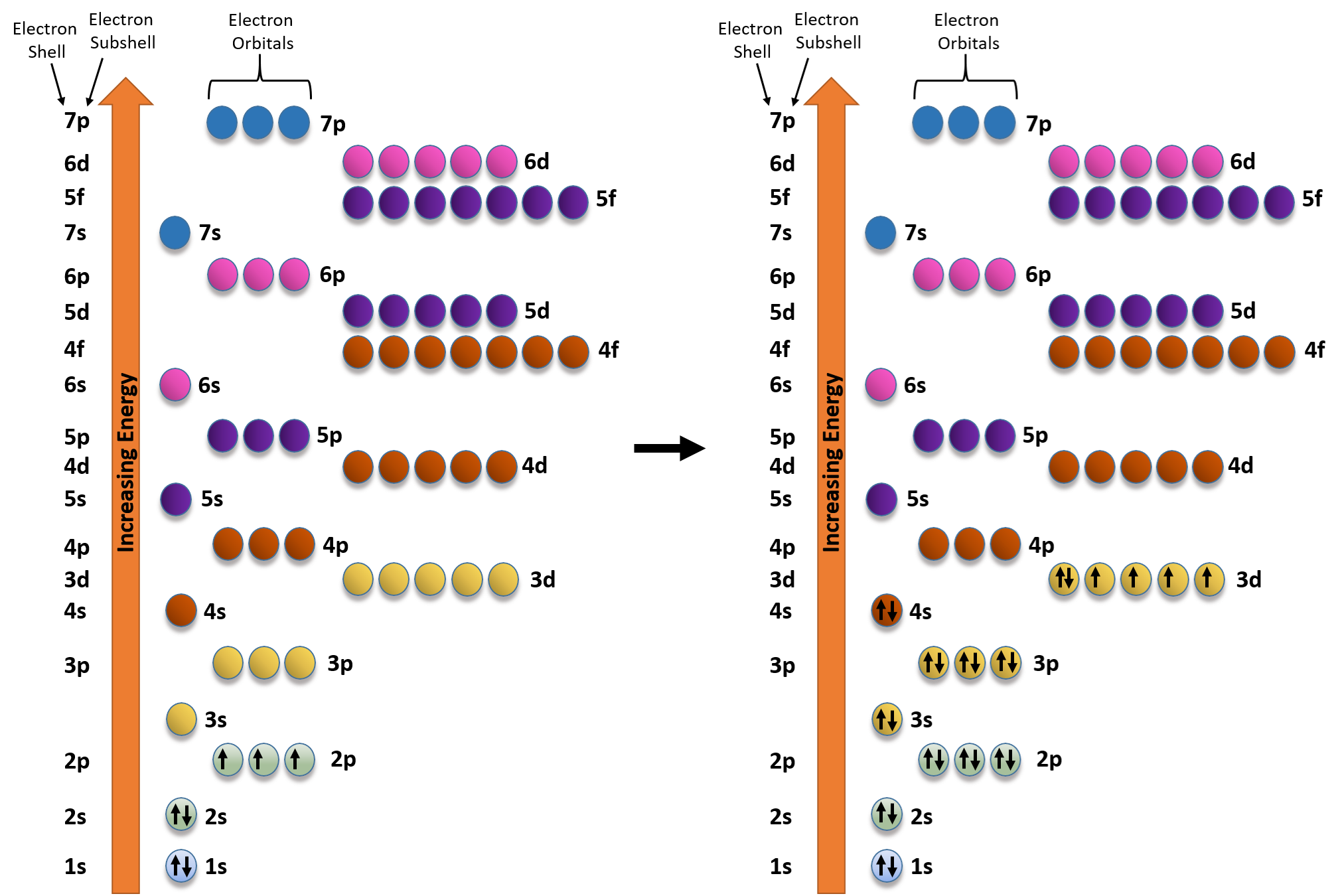
Web electronic iron configuration will be : Electron configurations describe where electrons are located around the nucleus of an atom. [ar]3d 6 4s 2 long form: Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: Iron, a.
Electron Configuration For Iron Iii Ion Rapid Electron
Electron configuration of neon (ne) [he] 2s 2 2p 6: The atomic number of iron is 26. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Web the electronic iron configuration (e.g.) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6, and the form [ ar ] 4s2 3d6 is abbreviated. 1s 2 2s 2 2p 3:
The Electron 2020 Give The Electron Configuration Of Chlorine
Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Once we have the configuration for fe, the ions are simple. Electron configuration of oxygen (o) [he] 2s 2 2p 4: Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Electron configuration through orbit (bohr principle) electron configuration through.
Open Iron Electron Configuration Free Transparent PNG Download PNGkey
Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web iron has 26 electrons so its normal electron configuration would be: Iron is a member of group.
Electron Configuration Of Iron Full worksheet today
Web the electron configuration of iron is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 , if the electron arrangement is through orbitals. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. We describe an electron configuration with a symbol that contains three pieces of information (.
Iron Periodic Table Electrons Review Home Decor
The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Once we have the configuration for fe, the ions are simple. Electron configuration of oxygen (o) [he] 2s 2 2p 4: [13] iron forms compounds mainly in the oxidation states +2. Web as an approximate rule, electron configurations are.
Iron Periodic Table Electrons Awesome Home
Web answer (1 of 3): Writing the electron configuration, you really only need the valence orbitals, and you can omit the core orbitals by notating it. Web the average quantity of iron in the human body is about 4.5 grams (about 0.004 percent), of which approximately 65 percent is in the form of hemoglobin, which transports molecular oxygen from the.
Write the full electron configuration of iron, YouTube
Iron is a member of group 8 and the first transition series of the periodic. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 shell structure: Web iron electron configuration short form: Web iron has 26 electrons so its normal electron configuration would be: The element symbol, fe, was shortened from the latin word '.
We Describe An Electron Configuration With A Symbol That Contains Three Pieces Of Information ( Figure 6.25 ):
Web iron has 26 electrons so its normal electron configuration would be: 1s 2 2s 2 2p 6: Electron configuration can be done in two ways. Electron configuration of neon (ne) [he] 2s 2 2p 6:
1S 2 2S 2 2P 3:
2 8 14 2 iron discovery discovery date: Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way. Web electronic iron configuration will be : Its valence orbitals are the 4s and 3d 's.
Web Its 26 Electrons Are Arranged In The Configuration [Ar]3D 6 4S 2, Of Which The 3D And 4S Electrons Are Relatively Close In Energy, And Thus A Number Of Electrons Can Be Ionized.
Both are explained in the video below. Web 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 to write an electron configuration for any element you'll need to apply the aufbau principle and use a diagonal diagram. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons).
And Most Of The Rest Stored In The Body (.
Its symbol is short for ferrum which is a latin term. Nonetheless, it wasn’t easy to find the reason why it was written as [ ar ] 3d6 4s2 instead in some periodic table. Web the average quantity of iron in the human body is about 4.5 grams (about 0.004 percent), of which approximately 65 percent is in the form of hemoglobin, which transports molecular oxygen from the lungs throughout the body; The atomic number of iron is 26.