Iron And Oxygen Gas React To Form Iron Iii Oxide
Iron And Oxygen Gas React To Form Iron Iii Oxide - Upon reacting with oxygen, iron will be oxidized to either. How many moles of fe2o3 is formed when 2 moles of oxygen gas react with 3 moles of iron? 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0. Assuming there is an excess of iron, how many moles. According to reaction, 4 moles of iron metal reacts with 3 moles of oxygen gas to give 2. Web the iron reacts with water and oxygen to form hydrated iron(iii) oxide, which we see as rust. Here is the word equation for the reaction: Web the general equation for this reaction is: If only 5.2 g of fe,o; The oxide layer do not passivate the surface.
This reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Assuming there is an excess of iron, how many moles. Web iron reacts with oxygen, o 2, forming fe(ii) and fe(iii) oxides. To obtain the oxide, a little fine iron powder is mixed in a ceramic pot with sodium nitrate. How many moles of fe2o3 is formed when 2 moles of oxygen gas react with 3 moles of iron? Web the general equation for this reaction is: Metal + oxygen → metal oxide. Web iron (ii, iii) oxide fe₃o₄ forms on the combustion of powdered iron in oxygen or in the air.
According to reaction, 4 moles of iron metal reacts with 3 moles of oxygen gas to give 2. Chemistry chemical reactions chemical reactions and. Metal + oxygen → metal oxide. Some metals will react with oxygen when they burn. Iron + water + oxygen → hydrated. Web science chemistry chemistry questions and answers iron and oxygen react to form iron (iii) oxide according to the chemical equation: Web iron (ii, iii) oxide fe₃o₄ forms on the combustion of powdered iron in oxygen or in the air. Web science chemistry oxygen gas reacts with iron to form iron(iii) oxide. Web what type of reaction is this: These reactions are called combustion reactions.
Solved Submit Part C Solid iron III) oxide reacts with
Web what type of reaction is this: Metal + oxygen → metal oxide. These reactions are called combustion reactions. According to reaction, 4 moles of iron metal reacts with 3 moles of oxygen gas to give 2. Powder or iron wool, can burn:
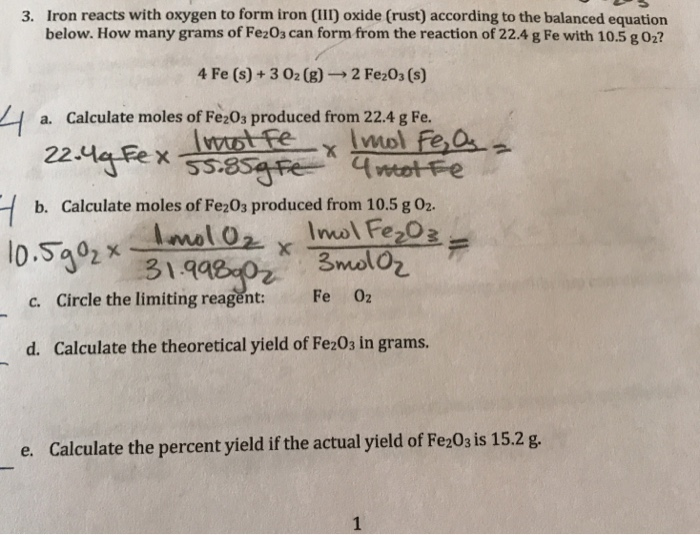
Solved 3. Iron reacts with oxygen to form iron (III) oxide
Web the general equation for this reaction is: Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass fe2o3 = 159.69 g/mol step 2: Some metals will react with oxygen when they burn. You know it is iron iii, not iron ii, because oxygen is. 4fe (s) + 3o2 (g).
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379
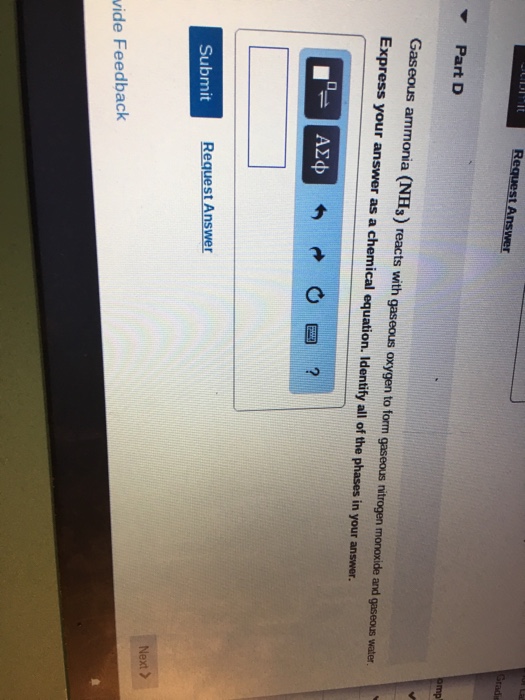
Iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. Web the general equation for this reaction is: Iron + water + oxygen → hydrated. Web science chemistry chemistry questions and answers iron and oxygen react to form iron (iii) oxide according to the chemical equation: Assuming there is an excess of iron,.
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Iron + water + oxygen → hydrated. Web iron (ii, iii) oxide fe₃o₄ forms on the combustion of powdered iron in oxygen or in the air. Study guides chemistry 19 cards to. Web what type of reaction is this:
Solved Iron reacts with oxygen to produce iron (III)
Here is the word equation for the reaction: Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass fe2o3 = 159.69 g/mol step 2: The oxide layer do not passivate the surface. Web what type of reaction is this: Web iron reacts with oxygen, o 2, forming fe(ii) and fe(iii).
PPT The Law of Conservation of Matter PowerPoint Presentation ID
If only 5.2 g of fe,o; Web what type of reaction is this: You know it is iron iii, not iron ii, because oxygen is. Web science chemistry chemistry questions and answers iron and oxygen react to form iron (iii) oxide according to the chemical equation: 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0.
when iron rusts, solid iron reacts with gaseous oxygen to form solid
To obtain the oxide, a little fine iron powder is mixed in a ceramic pot with sodium nitrate. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0. Study guides chemistry 19 cards to. Here is the word equation for the reaction: How many moles of fe2o3 is formed when 2 moles of oxygen gas react with 3 moles of.
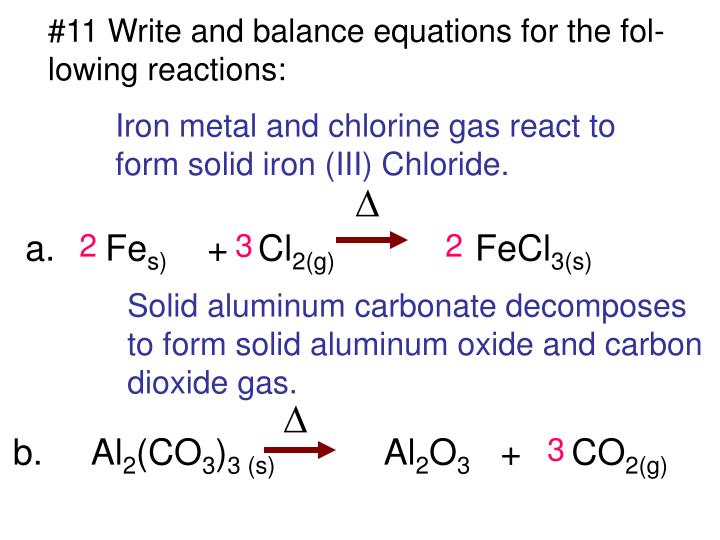
PPT 1. Write a sentence that describes this chemical reaction
Some metals will react with oxygen when they burn. You know it is iron iii, not iron ii, because oxygen is. Web iron (ii, iii) oxide fe₃o₄ forms on the combustion of powdered iron in oxygen or in the air. These reactions are called combustion reactions. Web the balanced chemical reaction between iron and oxygen gas is given by :
Blog Posts
Web science chemistry oxygen gas reacts with iron to form iron(iii) oxide. Web science chemistry chemistry questions and answers iron and oxygen react to form iron (iii) oxide according to the chemical equation: Powder or iron wool, can burn: Web iron reacts with oxygen to form iron (iii) oxide: These reactions are called combustion reactions.
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
If only 5.2 g of fe,o; Iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. Iron + water + oxygen → hydrated. According to reaction, 4 moles of iron metal reacts with 3 moles of oxygen gas to give 2. Chemistry chemical reactions chemical reactions and.
Web The Balanced Chemical Reaction Between Iron And Oxygen Gas Is Given By :
Study guides chemistry 19 cards to. According to reaction, 4 moles of iron metal reacts with 3 moles of oxygen gas to give 2. Web what type of reaction is this: Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide.
The Oxide Layer Do Not Passivate The Surface.
Upon reacting with oxygen, iron will be oxidized to either. This reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. Powder or iron wool, can burn: 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0.
Web Iron (Ii, Iii) Oxide Fe₃O₄ Forms On The Combustion Of Powdered Iron In Oxygen Or In The Air.
Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass fe2o3 = 159.69 g/mol step 2: You know it is iron iii, not iron ii, because oxygen is. Web the general equation for this reaction is: How many moles of fe2o3 is formed when 2 moles of oxygen gas react with 3 moles of iron?
Web The Iron Reacts With Water And Oxygen To Form Hydrated Iron(Iii) Oxide, Which We See As Rust.
Web science chemistry chemistry questions and answers iron and oxygen react to form iron (iii) oxide according to the chemical equation: Chemistry chemical reactions chemical reactions and. These reactions are called combustion reactions. Web science chemistry oxygen gas reacts with iron to form iron(iii) oxide.