How Many Hydrogen Bonds Can A Water Molecule Form
How Many Hydrogen Bonds Can A Water Molecule Form - Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Web answer (1 of 2): That means that every hydrogen. Web the dynamic interactions of water molecules. In h 2 o, only two of the six. Therefore one single water molecule does not contain any. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. One molecule of water can make four. Web the molecular formula for water is h 2 o.
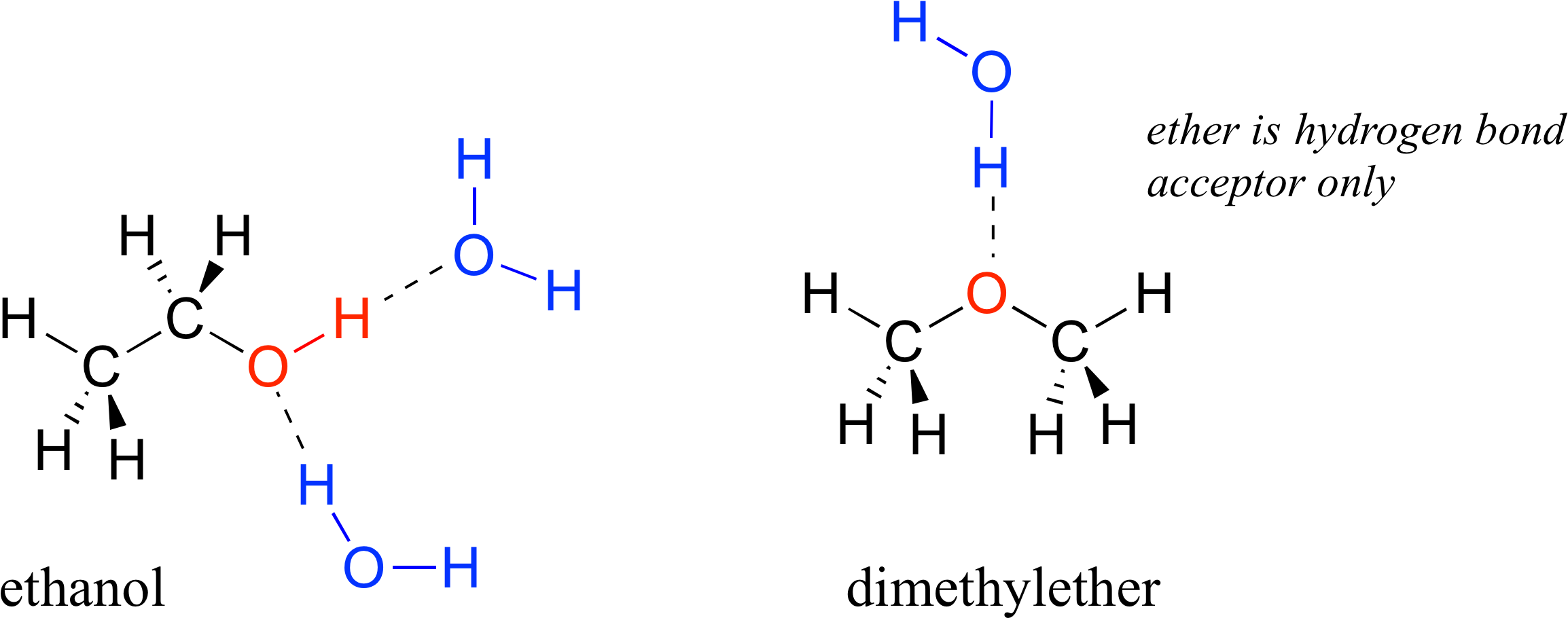
Web water is can ideally model of hydrogen stick. Therefore one single water molecule does not contain any. In h 2 o, only two of the six. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web a hydrogen bond will form between the negative oxygen of one molecule with the positive hydrogen of another molecule. Web answer (1 of 2): Hydrogen bond is an intermolecular force (force between molecules). Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule.
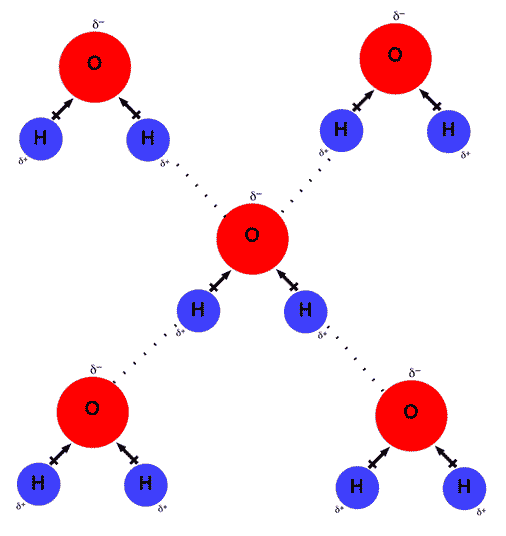
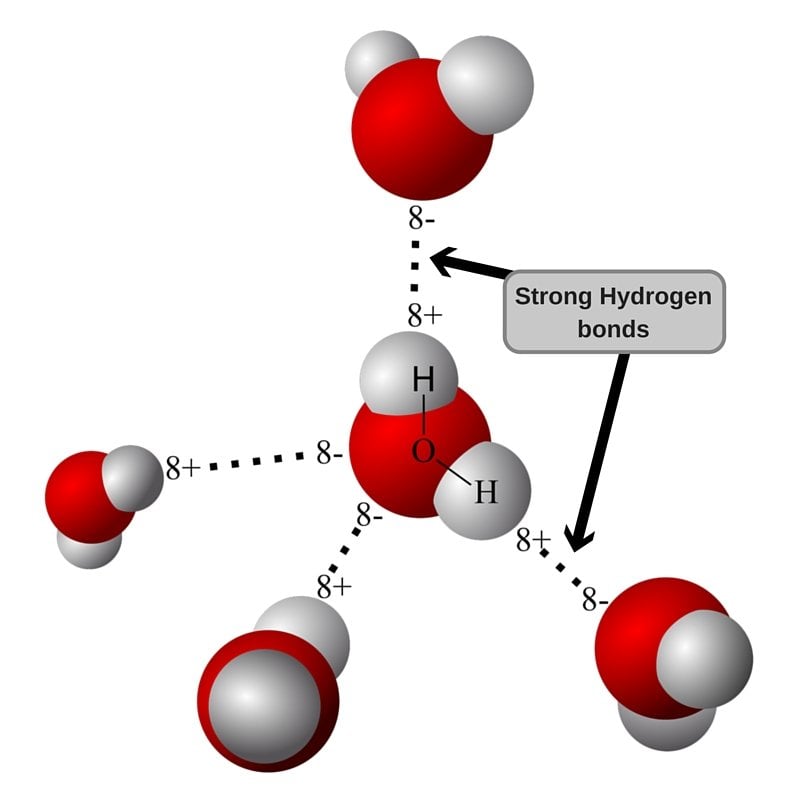
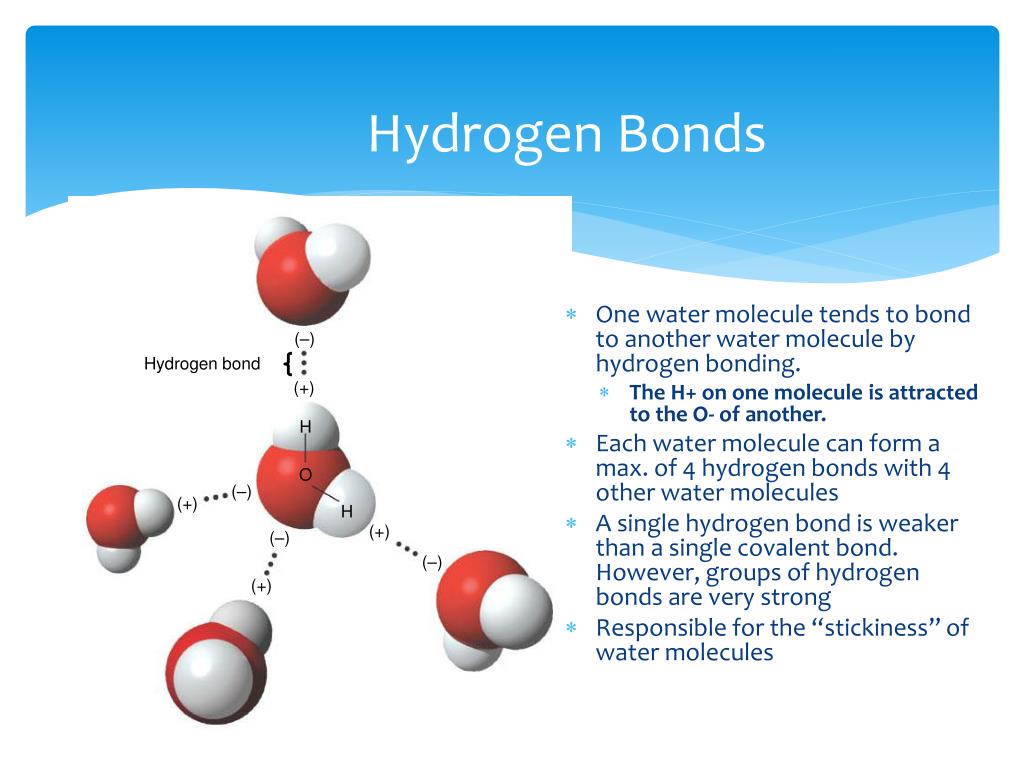
Web lewis structure of h 2 o indicating bond angle and bond length. Web answer (1 of 2): Each water molecule is surrounded by four neighboring h 2 os. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Hydrogen bond is an intermolecular force (force between molecules). Web a hydrogen bond will form between the negative oxygen of one molecule with the positive hydrogen of another molecule. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure.
😎 What properties of water make it essential to life. Why Water Is
One molecule of water can make four. Each water molecule is surrounded by four neighboring h 2 os. Hydrogen bond is an intermolecular force (force between molecules). That means that every hydrogen. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Both an oxygen atom and 2 hydrogen atoms in one molecule. Web water is can ideally model of hydrogen stick. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web water is capable of participating in 4 hydrogen.
How many hydrogen bonds are attached to each water molecule in a solid
Web a hydrogen bond will form between the negative oxygen of one molecule with the positive hydrogen of another molecule. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web water is can ideally model of hydrogen stick. Web the dynamic interactions of water molecules. In h 2 o, only two of the six.
Why Do Fingers/Hands Stick To Ice? » Science ABC
Web the molecular formula for water is h 2 o. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each molecules makes 2. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a.
PPT Properties of Water PowerPoint Presentation, free download ID
Therefore one single water molecule does not contain any. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Web the dynamic interactions of water molecules. One molecule of water can make four. Web up to 4 hydrogen bonds can form between a single water molecule.
Water
Therefore one single water molecule does not contain any. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web but if you take 100 water molecules and count how many hydrogen bonds there are between them, the answer will be about 200 because each.
Download How Many Hydrogen Bonds Can A Water Molecule Form Hydrogen
Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. In h 2 o, only two of the six. Web but if you take 100 water molecules and count how many hydrogen bonds.
Water Polar Covalent Bond My XXX Hot Girl
Web water is can ideally model of hydrogen stick. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web answer (1 of 2): Web water is capable of participating in 4 hydrogen bonds at once, granted it only.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Each water molecule is surrounded by four neighboring h 2 os. Therefore one single water molecule does not contain any. In h 2 o, only two of the six. Web in water, each hydrogen nucleus is covalently bound to the.
Pin on Hydrogen
Web answer (1 of 2): That means that every hydrogen. Web water is can ideally model of hydrogen stick. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of.
Web But If You Take 100 Water Molecules And Count How Many Hydrogen Bonds There Are Between Them, The Answer Will Be About 200 Because Each Molecules Makes 2.
One molecule of water consists of one oxygen atom covalently bonded to two hydrogen atoms. Each water molecule is surrounded by four neighboring h 2 os. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Web how many hydrogen bonds can a single water molecule form?
Web The Dynamic Interactions Of Water Molecules.
Web water is can ideally model of hydrogen stick. One molecule of water can make four. Therefore one single water molecule does not contain any. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules:
Web Water Is Unique Because Its Oxygen Atom Has Two Lone Pairs And Two Hydrogen Atoms, Meaning That The Total Number Of Bonds Of A Water Molecule Is Up To Four.
In h 2 o, only two of the six. Web answer (1 of 2): That means that every hydrogen. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°.
Web A Hydrogen Bond Will Form Between The Negative Oxygen Of One Molecule With The Positive Hydrogen Of Another Molecule.
Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure.