How Many Covalent Bonds Does Phosphorus Form
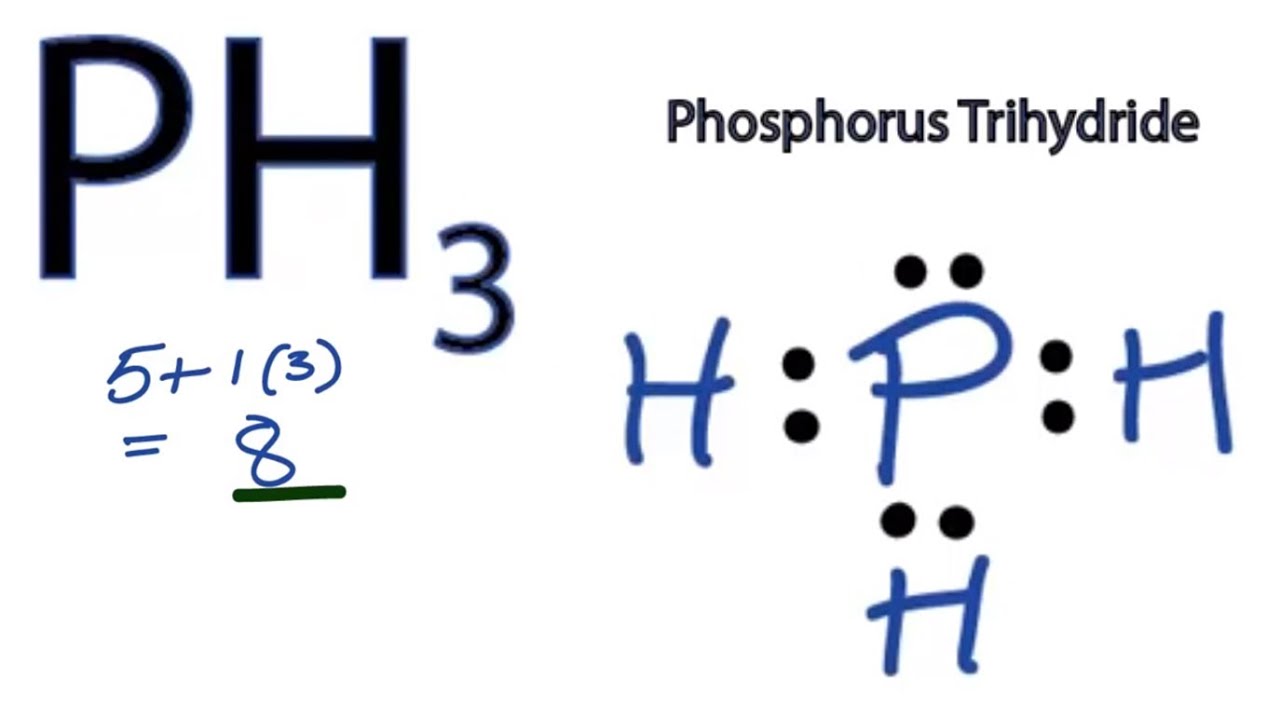
How Many Covalent Bonds Does Phosphorus Form - Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? So there are three covalent bonds and one lone pair of electrons on. This is summarized in the table below. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. And group 7a form one bond. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web how many bonds does phosphorus typically make?
Group 5a form 3 bonds; Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Web the formula of the carbonate ion is co 32−. So there are three covalent bonds and one lone pair of electrons on. Covalent bond two atoms form a covalent chemical bond. How many bonds does phosphorus typically make? Web typically, the atoms of group 4a form 4 covalent bonds; Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom?
Therefore phosphorus maximum covalency of 6. And group 7a form one bond. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web there are many different modifications of phosphorus in nature. Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web some compounds contain both covalent and ionic bonds.
Lewis Dot Diagram Phosphorus
Web there are many different modifications of phosphorus in nature. Web from the diagram, we can see that phosphorus has a full eight electrons in its outermost shell. And like a central silicon atom, there are four pairs of electrons being shared with. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as.
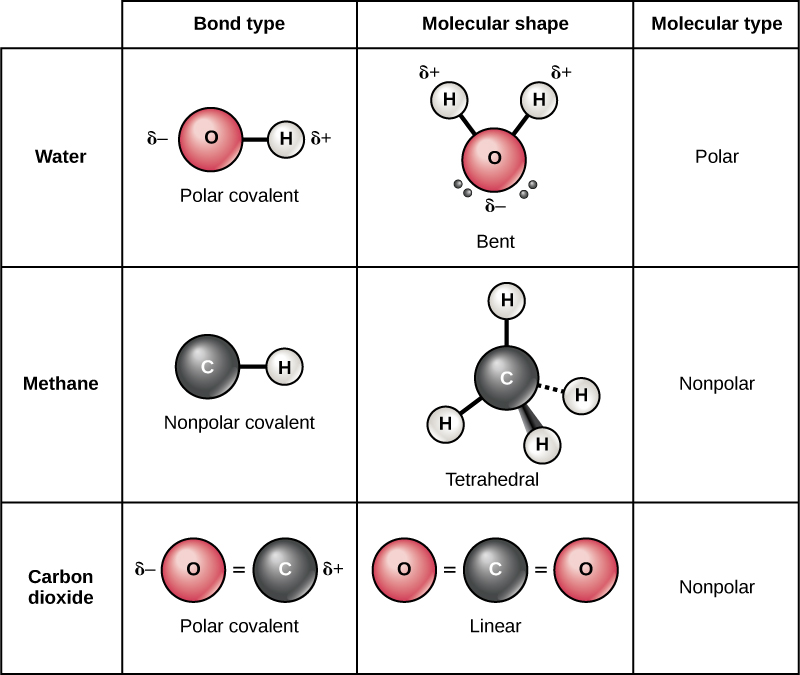
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. So.
Is SiO2 Ionic or Covalent? Techiescientist
Web the formula of the carbonate ion is co 32−. Web some compounds contain both covalent and ionic bonds. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. So there are three covalent bonds and one lone pair of electrons on. Web the most commonly observed (formal) oxidation.
FileElectron shell 015 Phosphorus.svg Wikimedia Commons Phosphorus
Web there are many different modifications of phosphorus in nature. So, phosphorous can exceed its valency from +3 to +5. So there are three covalent bonds and one lone pair of electrons on. Covalent bond two atoms form a covalent chemical bond. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web how many bonds does phosphorus typically make? So there are three covalent bonds and one lone pair of electrons on. Web the formula of the carbonate ion is co 32−. So there are three covalent bonds and one lone pair of electrons on. Web there are many different modifications of phosphorus in nature.
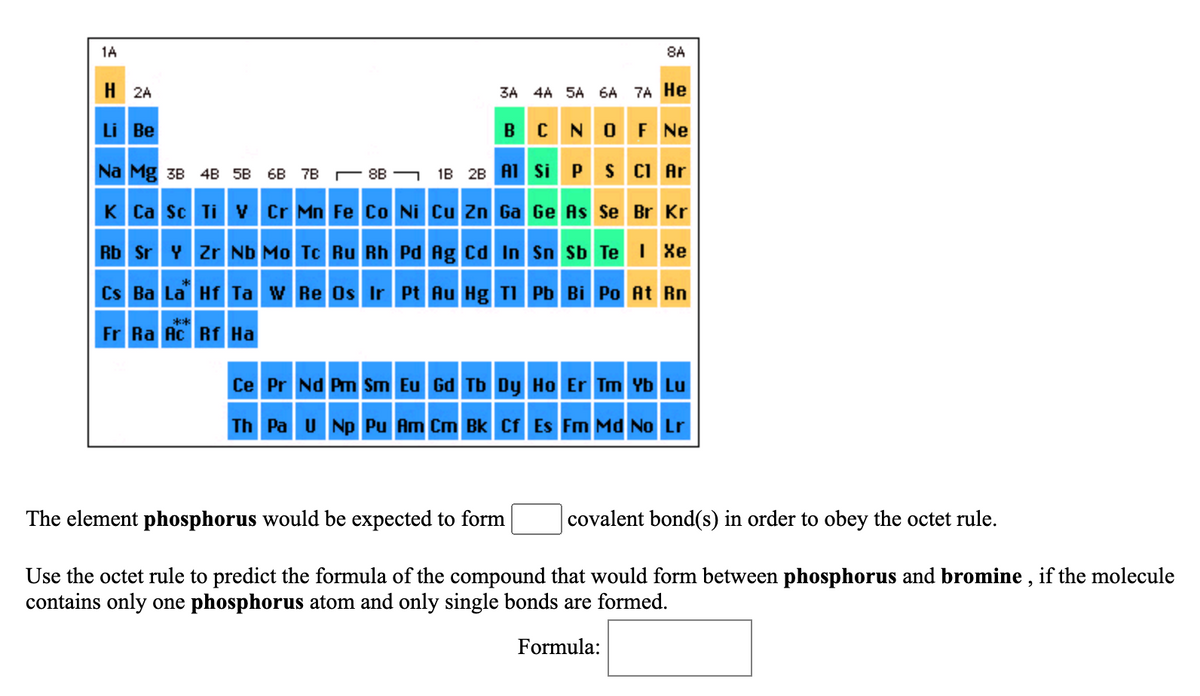
Answered The element phosphorus would be… bartleby
Web how many bonds does phosphorus typically make? Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web some compounds contain both covalent and ionic bonds. Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. This is summarized in the.
LabXchange
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although.
How does phosphorus form 5 covalent bonds? The Unconditional Guru
Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. So there are three covalent bonds and one lone pair of electrons on. How many bonds does phosphorus typically make? Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although.
How Many Single Bonds Can Carbon Form fredhughesdesign
Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. And group 7a form one bond. Web how many bonds does phosphorus typically make? So there are three covalent bonds and one lone pair of electrons on. Web the number of valence.
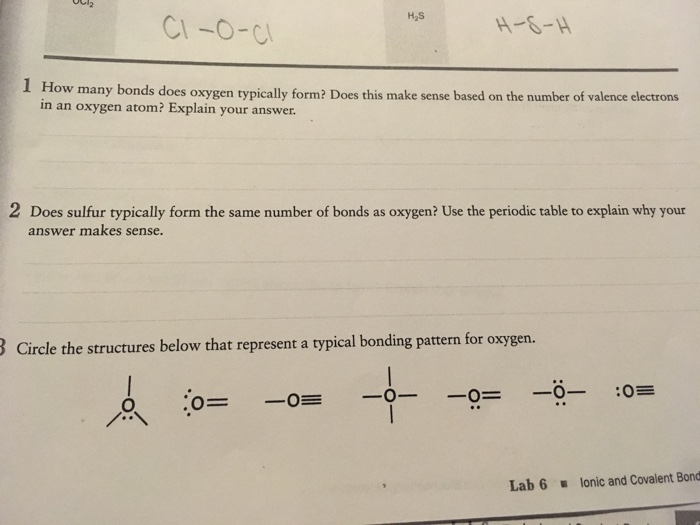
Solved H2S CIOC HSH 1 How many bonds does oxygen
Web correct option is b) phosphorous has an atomic number of 15 and electronic configuration as 1s 22s 22p 63s 23p 3. So there are three covalent bonds and one lone pair of electrons on. Group 6a form 2 bonds; Web some compounds contain both covalent and ionic bonds. Web how many bonds does phosphorus typically make?
So There Are Three Covalent Bonds And One Lone Pair Of Electrons On.
Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web in the pcl3 molecule, each chlorine is bonded by a single covalent bond with phosphorus. Web some compounds contain both covalent and ionic bonds. Web there are many different modifications of phosphorus in nature.
Web Correct Option Is B) Phosphorous Has An Atomic Number Of 15 And Electronic Configuration As 1S 22S 22P 63S 23P 3.
Group 6a form 2 bonds; And like a central silicon atom, there are four pairs of electrons being shared with. Web the most commonly observed (formal) oxidation state of phosphorus in molecules and crystals is +3 and +5, although a variety of oxidation states, from −3 to 4,. And group 7a form one bond.
Group 5A Form 3 Bonds;
Covalent bond two atoms form a covalent chemical bond. Web how many covalent bonds does a phosphorus atom normally form, based on the number of valence electrons in the atom? So there are three covalent bonds and one lone pair of electrons on. Web the formula of the carbonate ion is co 32−.
How Many Bonds Does Phosphorus Typically Make?
This is summarized in the table below. Therefore phosphorus maximum covalency of 6. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. So, phosphorous can exceed its valency from +3 to +5.