How Many Atoms Are Required To Form A Molecule
How Many Atoms Are Required To Form A Molecule - Often they lose or gain electrons to have the same number of electrons as the nearest. Group of two or more atoms held together by chemical bonds. Web atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. Web how many atoms are required to form a molecule? Groups of two or more atoms held together by chemical. Oxygen is the most common element by mass (43% of all. Web solution verified by toppr molecule: There are three atoms in a molecule of water. Web so, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. One oxygen atom and 2.
Web a molecule is made of two or more atoms chemically bonded together. An oxygen molecule ( o2 ) contains 2 oxygen atoms. There are three atoms in a molecule of water. Web solution verified by toppr molecule: Web how many atoms are required to form a molecule? At least two at least three at least four at least five o only one this problem has been solved! Using formulas to indicate how many atoms of each. So, minimum 2 atoms are required to form a molecule. Web because 1 h 2 molecule contains 2 h atoms, 1 mol of h 2 molecules (6.02 × 10 23 molecules) has 2 mol of h atoms. For example, a molecule of water (h2o) is made up of two hydrogen.
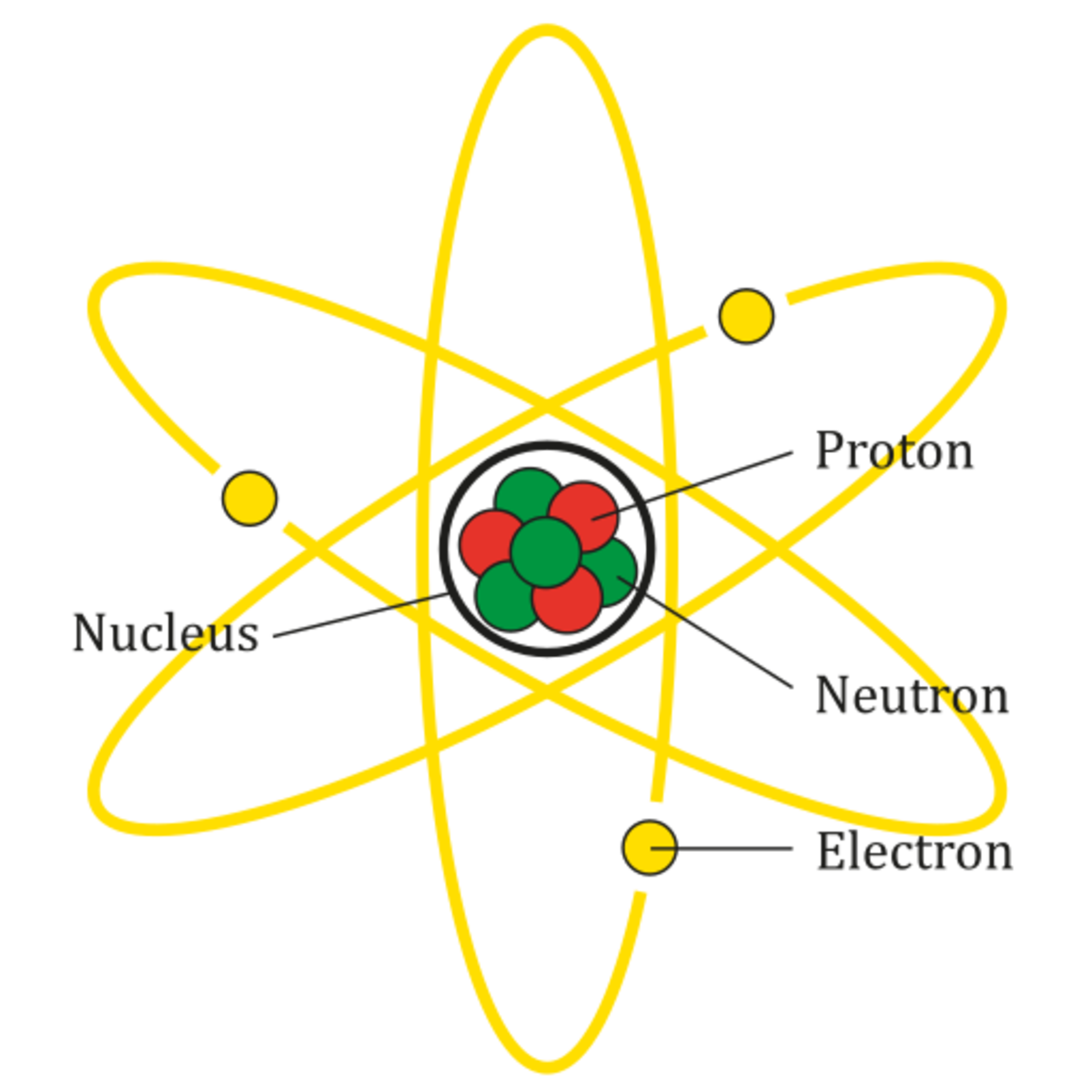
Groups of two or more atoms held together by chemical. Water is not an element but a molecule but it is the most common molecule in our bodies. Web since a mole is just a counting number, we are simply multiplying both values (1 molecule and 1 atoms) by avogadro's number to get a relationship in terms of moles. Web so, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. Atoms atoms are the building blocks of all matter. Web atoms, molecules, and ions ions • when atoms lose or gain electrons, they become ions. For example, a molecule of water (h2o) is made up of two hydrogen. And the number of atoms. Web because 1 h 2 molecule contains 2 h atoms, 1 mol of h 2 molecules (6.02 × 10 23 molecules) has 2 mol of h atoms. Web how many atoms are required to form a molecule?
Atoms, Molecules, and Compounds What's the Difference? Owlcation
The ability of atoms to attach and detach. Atoms atoms are the building blocks of all matter. Web so, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. The mole is related to the mass of an element in. Medium open in app solution verified by.
Types of Atoms Science at Your Doorstep
Web so, minimum 2 atoms are required to form a molecule. Noble gas elements exist as. Using formulas to indicate how many atoms of each. Web the number of atoms required to form a molecule depends on the type of molecule we are talking about. How many atoms does water make up?
Difference Between Molecule and Atom Compare the Difference Between
The mole is related to the mass of an element in. At least two at least three at least four at least five o only one this problem has been solved! Using formulas to indicate how many atoms of each. How many atoms does water make up? And the number of atoms.
About atoms and molecules Nature of the forces between them
Web since a mole is just a counting number, we are simply multiplying both values (1 molecule and 1 atoms) by avogadro's number to get a relationship in terms of moles. Noble gas elements exist as. An oxygen molecule ( o2 ) contains 2 oxygen atoms. Web so, minimum 2 atoms are required to form a molecule. So, minimum 2.
12 1 How Many Atoms
Web how many atoms are required to form a molecule? Web atoms, molecules, and ions ions • when atoms lose or gain electrons, they become ions. Web a molecule is made of two or more atoms chemically bonded together. Medium open in app solution verified by toppr molecule: Web so, minimum 2 atoms are required to form a molecule.
Atoms and Molecules Structure & Differences Embibe
Web so, minimum 2 atoms are required to form a molecule. Medium open in app solution verified by toppr molecule: Groups of two or more atoms held together by chemical. Electrons can be transferred from one atom to another. Web the number of atoms required to form a molecule depends on the type of molecule we are talking about.
chemistry picture
Groups of two or more atoms held together by chemical. Water is not an element but a molecule but it is the most common molecule in our bodies. Web how many atoms are required to form a molecule? Using formulas to indicate how many atoms of each. So, minimum 2 atoms are required to form a molecule.
Atoms and Molecules Chemistry Activities ⋆
For example, a molecule of water (h2o) is made up of two hydrogen. Web so, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. Groups of two or more atoms held together by chemical. So, minimum 2 atoms are required to form a molecule. Atoms atoms.
Molecules and Compounds Definition, Differenences [in Table Form]
Web how many atoms are required to form a molecule? Often they lose or gain electrons to have the same number of electrons as the nearest. Web so, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. There are three atoms in a molecule of water..
Groups Of Two Or More Atoms Held Together By Chemical.
Web because 1 h 2 molecule contains 2 h atoms, 1 mol of h 2 molecules (6.02 × 10 23 molecules) has 2 mol of h atoms. How many atoms does water make up? Medium open in app solution verified by toppr molecule: The number of atoms or other particles in a mole is the same for all substances.
Web How Many Atoms Are Required To Form A Molecule?
So, minimum 2 atoms are required to form a molecule. The ability of atoms to attach and detach. Noble gas elements exist as. The mole is related to the mass of an element in.
Web Since A Mole Is Just A Counting Number, We Are Simply Multiplying Both Values (1 Molecule And 1 Atoms) By Avogadro's Number To Get A Relationship In Terms Of Moles.
Often they lose or gain electrons to have the same number of electrons as the nearest. Web solution verified by toppr molecule: Web fun facts of measurement & math. Web a molecule is made of two or more atoms chemically bonded together.
Web How Many Atoms Are Required To Form A Molecule?
There are three atoms in a molecule of water. Electrons can be transferred from one atom to another. Web atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. Using formulas to indicate how many atoms of each.


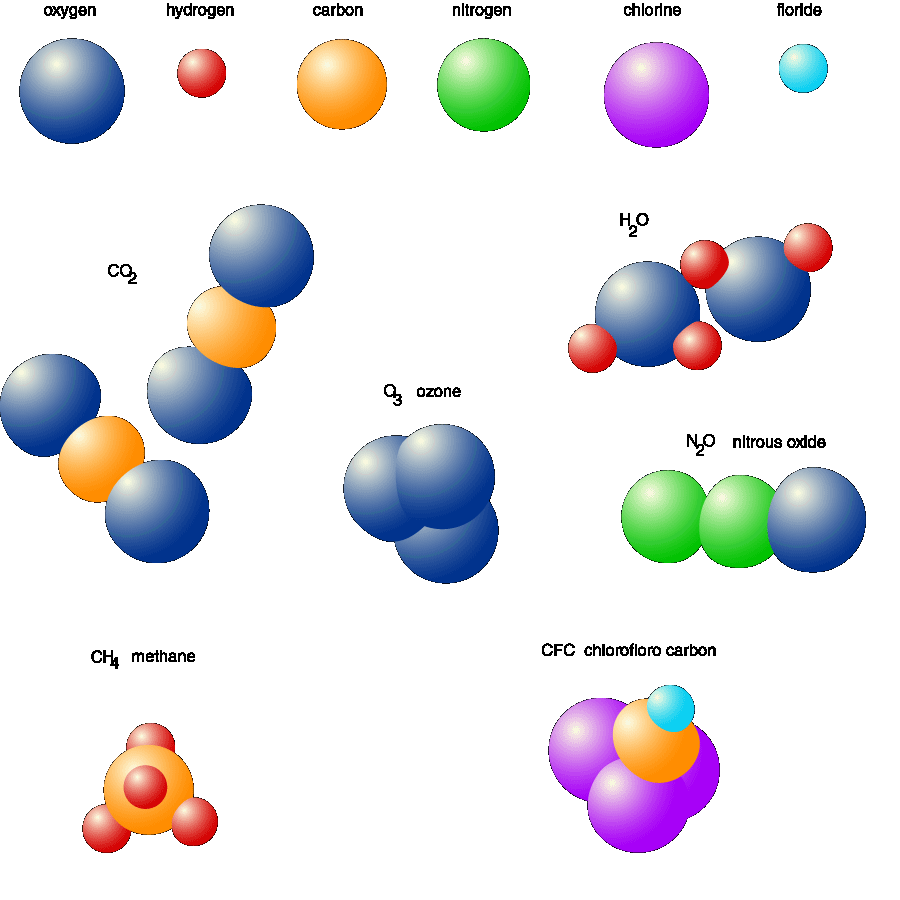




![Molecules and Compounds Definition, Differenences [in Table Form]](https://d1avenlh0i1xmr.cloudfront.net/large/756bdbc0-0026-418f-bc9f-de699cc72183/molecules-of-single-element-and-their-atomicity-teachoo-01.jpg)
