How Does A Positive Ion Form
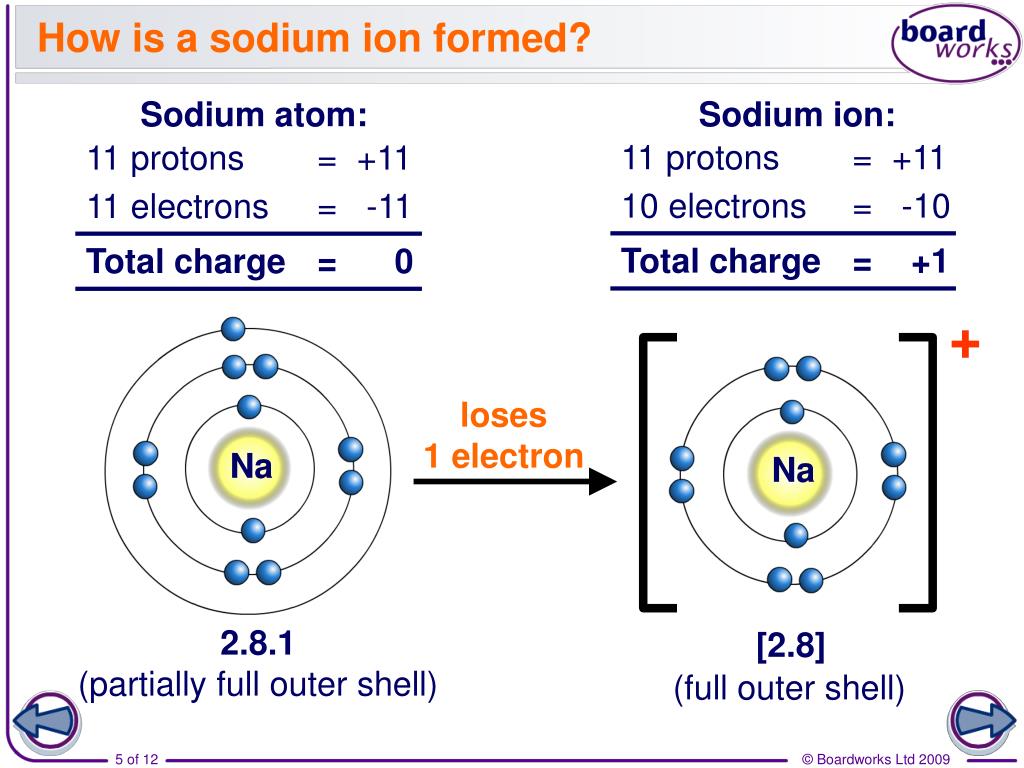
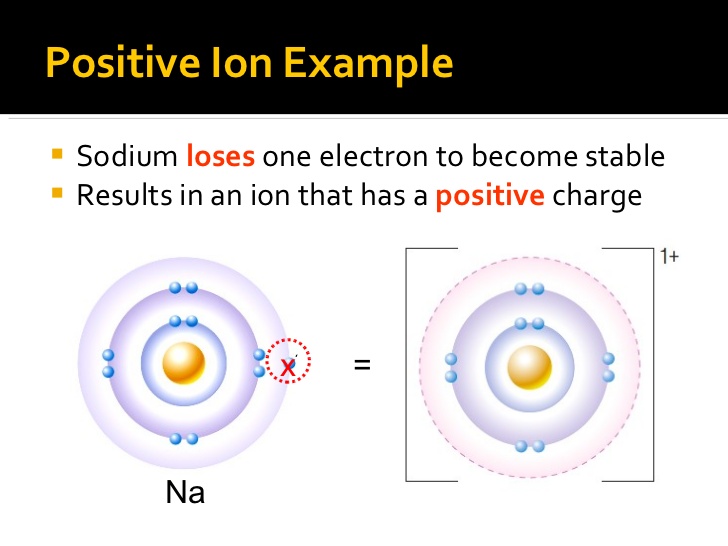
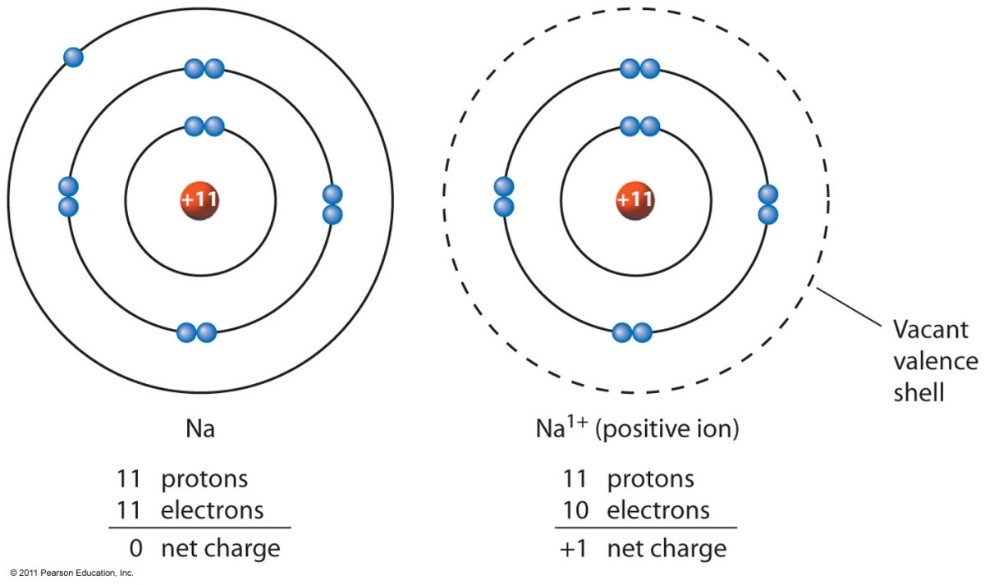
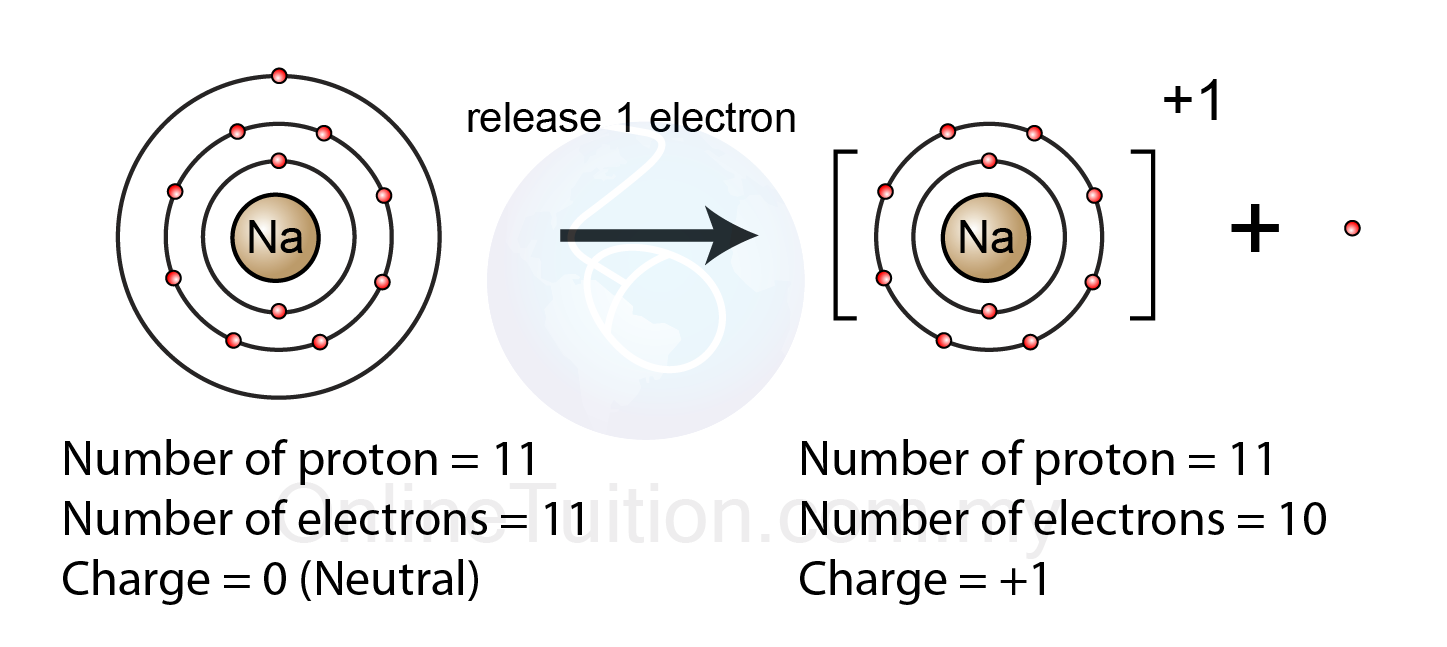
How Does A Positive Ion Form - Web forming positive ions metal atoms lose electrons from their outer shell when they form ions: An ion is a charged atom or molecule. Although the number of protons does not change in the ion, there is an excess number of protons over electrons which produces the positive charge. Web cations are the positive ions formed by the loss of one or more electrons. For example, a neutral sodium atom contains electrons in three main energy levels, n=1, n=2, n=3. • ( 18 votes) upvote flag ryan w 6 years ago an ion is an atom or molecule that has a different number of electrons than protons, so it has a charge. Web at r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. For example, let's look at lithium and fluorine: An ion is an atom or molecule with a net electrical charge. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges.
It has one valence electron in the n = 3 energy level. Electrostatics explains why this happens: All electrons in the outer energy level are lost. Web a positive ion is formed by the loss of negatively charged electrons. Web a positive ion is formed when an atom of a metal loses one or more electrons. To form a negative ion it must gain the electrons lost by the cation. When oppositely charged ions are brought They form through ionic bonding. These oppositely charged ions attract each other to form ionic networks (or lattices ). • ( 18 votes) upvote flag ryan w 6 years ago an ion is an atom or molecule that has a different number of electrons than protons, so it has a charge.
For example, let's look at lithium and fluorine: Examples of positive ions positive ions are typically metals or act like metals. The most commonly formed cations of the representative elements are those that involve the loss of all of the valence electrons. The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. • ( 18 votes) upvote flag ryan w 6 years ago an ion is an atom or molecule that has a different number of electrons than protons, so it has a charge. It has one electron in its valence shell, which makes it unstable. Electrons in the outer level. Consider the alkali metal sodium ( na). Lithium has 3 electrons, so. The ions are positive, because they have more protons than electrons the ions formed have.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
To form a negative ion it must gain the electrons lost by the cation. Web forming positive ions metal atoms lose electrons from their outer shell when they form ions: All electrons in the outer energy level are lost. Electrostatics explains why this happens: Web a positive ion forms when an atom loses one or more valence electron.
Ions
Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion. Examples of positive ions positive ions are typically metals or act like metals. Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. Web.
Molecular and Ionic Compounds Chemistry I
Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. Web cations are the positive ions formed by the loss of one or more electrons. The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. Web a positive ion is formed by.
NH4+ Lewis Structure (Ammonium Ion) Math, Lewis, Positivity
What is an example of a positive ion? It has one electron in its valence shell, which makes it unstable. Consider the alkali metal sodium ( na). They form through ionic bonding. These ions are positive because they contain more protons.
What happens if an atom is not neutral? Socratic
The transfer and sharing of electrons among atoms govern the chemistry of the. An ion is an atom or molecule with a net electrical charge. Ions migrate under the influence of an electrical field and are the conductors of electric current in electrolytic cells. The charge of an electron is considered to be negative by convention and this charge is.
Ions Types, Summary, Classification & Facts
4 comments ( 106 votes) upvote downvote flag The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. Many common materials contain these ions. Examples of positive ions positive ions are typically metals or act like metals. Electrostatics explains why this happens:
5.2.1 Formation of Ion Revision.my
These oppositely charged ions attract each other to form ionic networks (or lattices ). Consider the alkali metal sodium ( na). Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion. The degree of ionization of the plasma depends strongly on the electron.
What Does Ion Mean? Slanguide
An ion is an atom or molecule with a net electrical charge. 4 comments ( 106 votes) upvote downvote flag Web a positive ion forms when an atom loses one or more valence electron. Electrons in the outer level. Web a positive ion is formed when an atom of a metal loses one or more electrons.
Ions — Definition & Overview Expii
This gives the metal ion a filled valence shell. 4 comments ( 106 votes) upvote downvote flag For example, a neutral sodium atom contains electrons in three main energy levels, n=1, n=2, n=3. Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion..
3.2 Ions The Basics of General, Organic, and Biological Chemistry
The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. An ion is an atom or molecule with a net electrical charge. An ion is a charged atom or molecule. It has one electron in its valence shell, which makes it unstable. Although the number of protons does not change in.
Web At R0, The Ions Are More Stable (Have A Lower Potential Energy) Than They Are At An Infinite Internuclear Distance.
An atom can acquire a positive charge or a negative charge depending on whether the number of electrons Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion. It has one electron in its valence shell, which makes it unstable. When oppositely charged ions are brought
Web A Positive Ion Forms When An Atom Loses One Or More Valence Electron.
The most commonly formed cations of the representative elements are those that involve the loss of all of the valence electrons. It has one valence electron in the n = 3 energy level. Web cations are the positive ions formed by the loss of one or more electrons. This gives the metal ion a filled valence shell.
The Charge Of An Electron Is Considered To Be Negative By Convention And This Charge Is Equal And Opposite To The Charge Of A Proton, Which Is Considered To Be Positive By Convention.
Consider the alkali metal sodium ( na). Opposite charges attract and like charges repel. Although the number of protons does not change in the ion, there is an excess number of protons over electrons which produces the positive charge. Web formation of ions in ordinary chemical reactions, the nucleus of each atom (and thus the element's identity) remains unchanged.
Web Ion, Any Atom Or Group Of Atoms That Bears One Or More Positive Or Negative Electrical Charges.
The transfer and sharing of electrons among atoms govern the chemistry of the. Lithium has 3 electrons, so. 4 comments ( 106 votes) upvote downvote flag The ions are positive, because they have more protons than electrons the ions formed have.