Fda Form 3537
Fda Form 3537 - Use the following instructions to download the form if you encounter an. Addition of unique facility identifier (ufi) field in section 2. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web omb burden statement fda form 3537/3537a. Agent identification number in section 7; If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Instructions are available on the form. Create a new form 3537 please read and understand the information below. Online registration of food facilities.
Web omb burden statement fda form 3537/3537a. Use the following instructions to download the form if you encounter an. While the fda expects all registrants to provide their duns number with their registration or. Create a new form 3537 please read and understand the information below. Field appears upon user selecting ‘yes’ option. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Online registration of food facilities. If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Addition of unique facility identifier (ufi) field in section 2.
If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Field appears upon user selecting ‘yes’ option. Fda strongly encourages electronic registration via the internet, which. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. While the fda expects all registrants to provide their duns number with their registration or. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Online registration of food facilities. Addition of unique facility identifier (ufi) field in section 2. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537.
Form FDA 3537 Fill Out and Sign Printable PDF Template signNow
Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. While the fda expects all registrants to provide their duns number with their registration or. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Addition of unique facility identifier.
36 Fda Forms And Templates free to download in PDF
Instructions are available on the form. Online registration of food facilities. Field appears upon user selecting ‘yes’ option. Web omb burden statement fda form 3537/3537a. Create a new form 3537 please read and understand the information below.
Instrucciones FORM 3537 Instructions for Filling Out DHHS/FDA Forms
If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Web omb burden statement fda form 3537/3537a. While the fda expects all registrants to provide their duns number.
Form FDA 3537a DHHS/FDA Cancellation of Food Facility Registration
While the fda expects all registrants to provide their duns number with their registration or. Field appears upon user selecting ‘yes’ option. Create a new form 3537 please read and understand the information below. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. You may obtain a copy of this form by.
FDA FORM 3514 PDF
Instructions are available on the form. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Field appears upon user selecting ‘yes’ option..
Form FDA 3632 Product Reports on Lasers and Products Containing
Instructions are available on the form. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. Agent identification number in section 7; Use the following instructions to download the form if you encounter an. Field appears upon user selecting ‘yes’ option.
Form FDA 3356 Establishment Registration and Listing for HCT/Ps Free
Use the following instructions to download the form if you encounter an. Field appears upon user selecting ‘yes’ option. Addition of unique facility identifier (ufi) field in section 2. Agent identification number in section 7; Food and drug administration, center for food safety and applied nutrition, 5001 campus dr.
Why is the 2253 FDA Form Comments Field Not Populated in Vault? Veeva
You may obtain a copy of this form by writing to the u.s. Fda strongly encourages electronic registration via the internet, which. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Agent identification number in section 7; Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537.
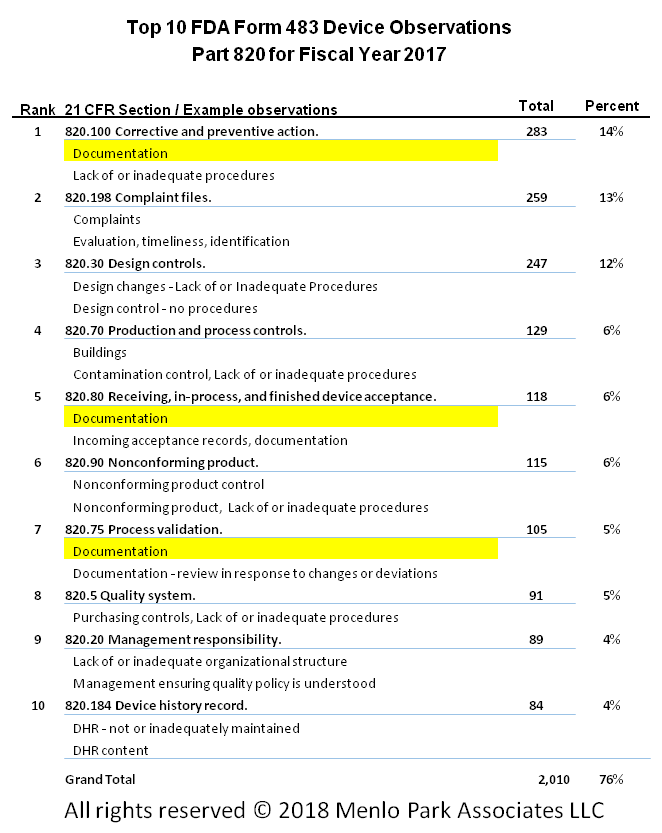
Menlo Park Software 100 Perfect Compliance
Instructions are available on the form. Web (1) you must register or renew a registration (including abbreviated registration renewals) using form fda 3537. Field appears upon user selecting ‘yes’ option. Fda strongly encourages electronic registration via the internet, which. Online registration of food facilities.
Fda 766 Fill Online, Printable, Fillable, Blank pdfFiller
Instructions are available on the form. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr. Addition of unique facility identifier (ufi) field in section 2. While the fda expects all registrants to provide their duns number with their registration or. Agent identification number in section 7;
Web (1) You Must Register Or Renew A Registration (Including Abbreviated Registration Renewals) Using Form Fda 3537.
Online registration of food facilities. Addition of unique facility identifier (ufi) field in section 2. Create a new form 3537 please read and understand the information below. Food and drug administration, center for food safety and applied nutrition, 5001 campus dr.
Use The Following Instructions To Download The Form If You Encounter An.
While the fda expects all registrants to provide their duns number with their registration or. Agent identification number in section 7; If you have any questions before registering or you feel your facility may be exempt, please send to ftn_fda_ftn@corp.ds.fedex.com or call 1.800.249.2953 (us & canada) or 1.716.879.1075 (international). Fda strongly encourages electronic registration via the internet, which.
Depending On The Browser You Are Using, You May Need To Download The Form To Enable Field Fillable Functionality.
Web omb burden statement fda form 3537/3537a. Instructions are available on the form. Web if you submit a registration or registration renewal by mail or fax, you must use the paper version of form fda 3537. You may obtain a copy of this form by writing to the u.s.