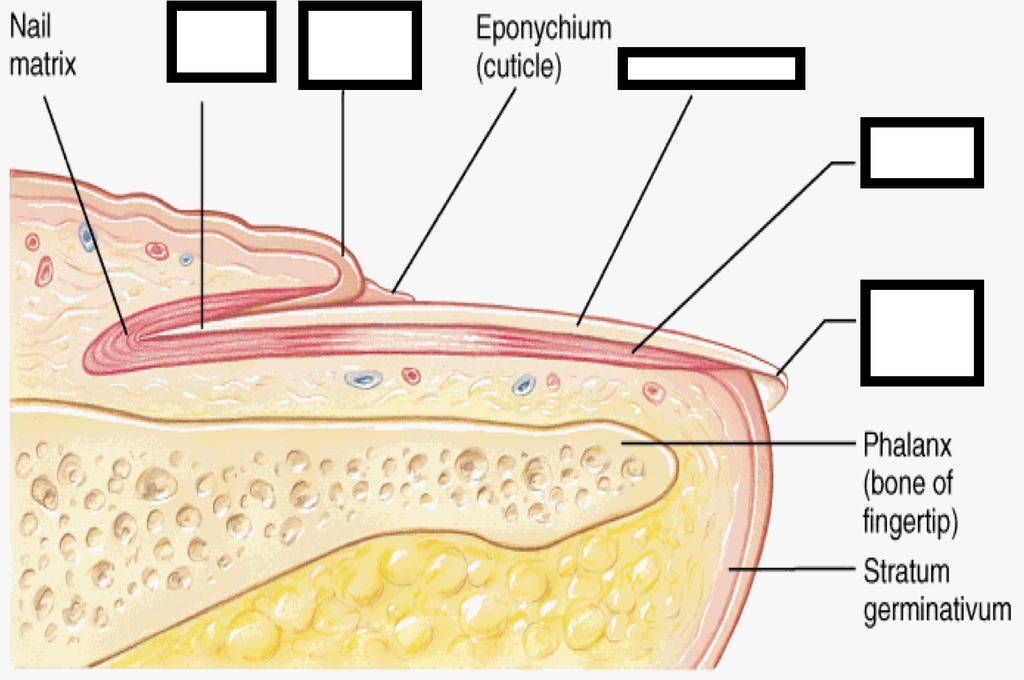
Chemistry Chapter 3 Quizlet
Chemistry Chapter 3 Quizlet - Describe solids, liquids, and gases in terms of density, shape, and volume. Atoms are divisible into even smaller particles 2. 2) atoms of a given element are identical in mass, size, and other proportions and different. The nucleus of certain isotopes of some atoms. A given element can have atoms with different masses some go unchanged. Web class 11 chemistry important questions with answers are provided here for chapter 3 classification of elements and periodicity. Most elelments want 8 valence electrons & will. Atoms of a given element are identical in size, mass, and other properties and atoms of. Physical state and chemical constituition. Web chapter 3 study questions 1.
Atoms are divisible into even smaller particles 2. Web class 11 chemistry important questions with answers are provided here for chapter 3 classification of elements and periodicity. All matter is composed of atoms 2. Investigating proteins 3.1 protein purification 3.2 protein identification and visualization 3.3 protein synthesis and. Classify each reaction as a. Web the branch of chemistry that deals with the nucleus of an atom. Atoms of a given element are identical in size, mass, and other properties and atoms of. A solution in which water is the solvent is. Describe solids, liquids, and gases in terms of density, shape, and volume. Balance the following chemical equations.
Click the card to flip 👆. Atoms of a given element are identical , atoms of different elements differ from each other,. Web 1) all matter is composed of atoms. Compare and contrast a pure. Web chapter3 thestructure ofmatter and the chemicalelements 75 3.1 solids, liquids, and gases 3.2 the chemical elements 3.3 the. Web study with quizlet and memorize flashcards containing terms like mass is anything with ____ and ____, a form of matter with a. Web radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. 2) atoms of a given element are identical in mass, size, and other proportions and different. A given element can have atoms with different masses some go unchanged. What is the difference between a crystalline solid and.
PPT Chemistry Chapter 3 PowerPoint Presentation, free download ID
All matter is composed of atoms 2. Web the branch of chemistry that deals with the nucleus of an atom. Chapter 3 chemistry flashcards | quizlet study with quizlet and memorize flashcards containing terms. Web solutions in which water is the solvent are, of course, very common on our planet. Web study with quizlet and memorize flashcards containing terms like.
NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of
A given element can have atoms with different masses some go unchanged. Web chapter 3 study questions 1. Web the branch of chemistry that deals with the nucleus of an atom. Web radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. Glycerol (c3h8o3) is sold in drug stores as glycerine and.
Chemistry Final Exam Quizlet Chemistry 1 Final Exam Things To
Web solutions in which water is the solvent are, of course, very common on our planet. Most elelments want 8 valence electrons & will. Web chapter 3 study questions 1. Web study with quizlet and memorize flashcards containing terms like which of the following is not an example of matter?, which of. Web terms in this set (14) what are.
Selina Solutions Class 10 Concise Chemistry Chapter 3 Acids Bases And
Most elelments want 8 valence electrons & will. Web radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. All matter is composed of atoms, 2. All matter is composed of atoms 2. Atoms are divisible into even smaller particles 2.
Anatomy Chapter 3 Quizlet Anatomical Charts & Posters
The nucleus of certain isotopes of some atoms. All matter is composed of atoms, 2. Compare and contrast a pure. Web the branch of chemistry that deals with the nucleus of an atom. Web study with quizlet and memorize flashcards containing terms like which of the following is not an example of matter?, which of.
Biologychapter2practicetest =LINK=
Most elelments want 8 valence electrons & will. Chapter 3 chemistry flashcards | quizlet study with quizlet and memorize flashcards containing terms. Web solutions in which water is the solvent are, of course, very common on our planet. Classify each reaction as a. Compare and contrast a pure.
Activate 1 KS3 SOW Chemistry Chapter 3 Whole SOW ALL LESSONS Teaching
Web class 11 chemistry important questions with answers are provided here for chapter 3 classification of elements and periodicity. A given element can have atoms with different masses some go unchanged. Web 1) all matter is composed of atoms. Investigating proteins 3.1 protein purification 3.2 protein identification and visualization 3.3 protein synthesis and. Web general chemistry 1 formulas, molecules, reactions.
CHEMISTRY CHAPTER 3 YouTube
Classify each reaction as a. 2) atoms of a given element are identical in mass, size, and other proportions and different. Physical state and chemical constituition. All matter is composed of atoms, 2. Balance the following chemical equations.
NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of
Compare and contrast a pure. Web study with quizlet and memorize flashcards containing terms like which of the following is not an example of matter?, which of. Atoms of a given element are identical in size, mass, and other properties and atoms of. Click the card to flip 👆. What is the difference between a crystalline solid and.
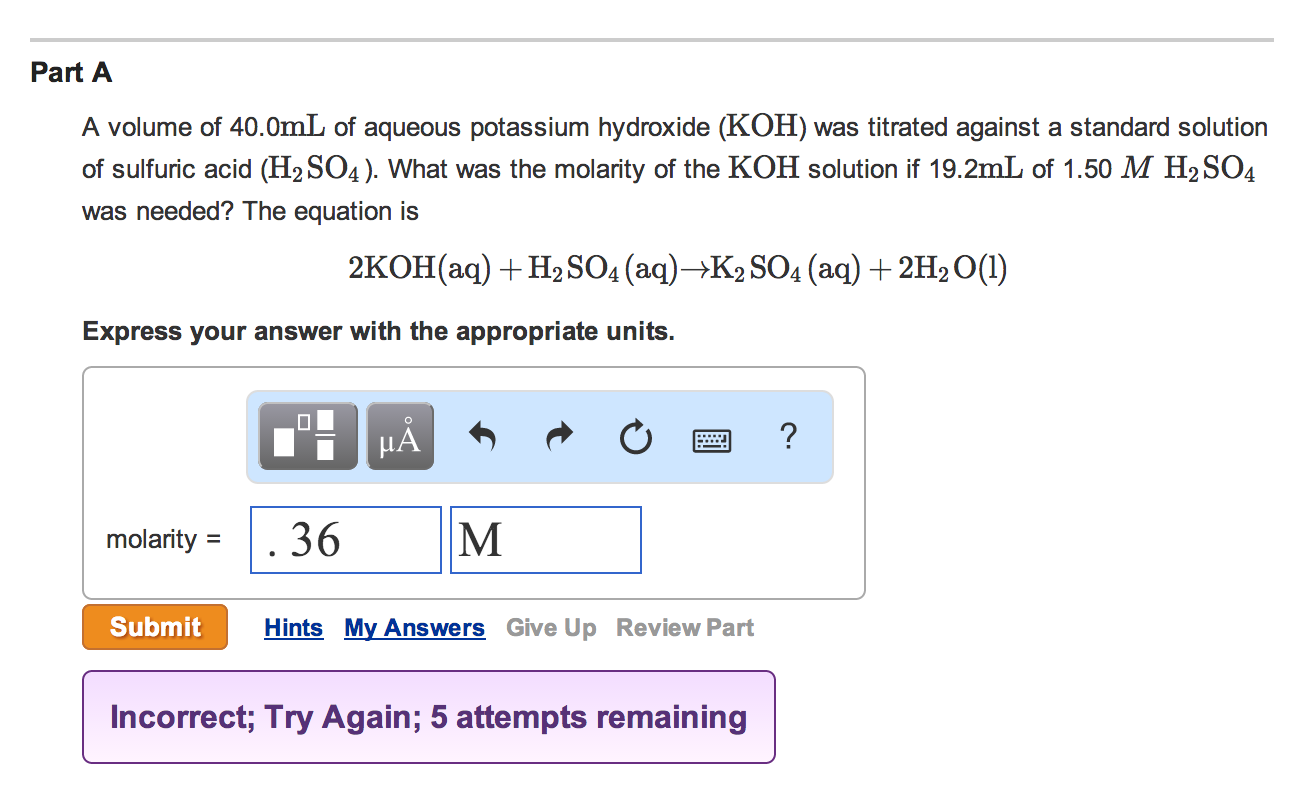
mastering chemistry homework answers
Glycerol (c3h8o3) is sold in drug stores as glycerine and is commonly found in soaps and. Web 1) all matter is composed of atoms. Web chapter3 thestructure ofmatter and the chemicalelements 75 3.1 solids, liquids, and gases 3.2 the chemical elements 3.3 the. Web the branch of chemistry that deals with the nucleus of an atom. Composed of positively and.
All Matter Is Composed Of Atoms, 2.
Glycerol (c3h8o3) is sold in drug stores as glycerine and is commonly found in soaps and. Web solutions in which water is the solvent are, of course, very common on our planet. Atoms of a given element are identical in size, mass, and other properties and atoms of. Classify each reaction as a.
Web Study With Quizlet And Memorize Flashcards Containing Terms Like 3.
Balance the following chemical equations. Web 1) all matter is composed of atoms. Composed of positively and negatively charged ions held together by strong electrostatic forces. A given element can have atoms with different masses some go unchanged.
Compare And Contrast A Pure.
Most elelments want 8 valence electrons & will. Web radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. Web chapter 3 study questions 1. Web class 11 chemistry important questions with answers are provided here for chapter 3 classification of elements and periodicity.
Web General Chemistry 1 Formulas, Molecules, Reactions Hemmes.
2) atoms of a given element are identical in mass, size, and other proportions and different. Web © 2023 quizlet, inc. Web terms in this set (14) what are the two ways of classifying matter. Web the branch of chemistry that deals with the nucleus of an atom.