Chapter 7 Chemical Formulas And Chemical Compounds
Chapter 7 Chemical Formulas And Chemical Compounds - Number of atoms of each element in one molecule of a compound c. Relative number of atoms of each kind in a chemical formula. Label each of the following statements as true or false: Valence electrons and oxidation state. C) type of bond holding particles together in a compound. Chemical formula writing worksheet review with answer key.pdf. Names & formulas of chemical compounds jun 25 2023 learn and review on the go! Chemical compounds and chemical formulas. Total number of positive and negative charges must be equal. Web learn how to name binary and polyatomic ionic compounds, and see some examples of naming these types of compounds.
Ions formed from 1 atom. Naming ionic and covalent compounds. Chemical compounds and chemical formulas. Web chapter 7 practice test with answer key.pdf. B) number of atoms or ions in a compound. Web key terms monatomic ion oxyanion binary compound salt nomenclature the total number of natural and synthetic chemical compounds runs in the millions. Common chemicals and their common names. Web get faster at matching terms created by trina2017 terms in this set (48) hydrocarbons molecular compounds composed solely of carbon and hydrogen monatomic ions ions formed from a single atom stock system system of naming chemical ions and elements binary compound a compound. Total number of positive and negative charges must be equal. Label each of the following statements as true or false:
Web key terms monatomic ion oxyanion binary compound salt nomenclature the total number of natural and synthetic chemical compounds runs in the millions. Naming ionic and covalent compounds. For a molecular compound, the chemical formula reveals the number of atoms of each element contained in a single molecule of the compound. B) number of atoms or ions in a compound. Names & formulas of chemical compounds jun 25 2023 learn and review on the go! Represents the simplest whole number ratio of the compounds. Relative number of atoms of each kind in a chemical formula. The empirical formula for a compound shows the symbols of the elements with subscripts indicating the. Number of atoms of each element in one molecule of a compound c. Chemical formulas and chemical compounds.
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
What is the oxidation number of the atoms in these formulas? Represents the simplest whole number ratio of the compounds. Number of atoms of each element in one molecule of a compound c. What is true about the charges in a binary compound. Common chemicals and their common names.
PPT Chapter 7 Chemical Formulas and Names PowerPoint Presentation
The number of atoms of each element contained in a single molecule of the compound. Number of atoms of each element in one molecule of a compound c. So the sulfur (“s”) is 6+. Web a) charge on an ion. The empirical formula for a compound shows the symbols of the elements with subscripts indicating the.
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Represents the simplest whole number ratio of the compounds. D) distribution of electrons among the bonded particles in a compound. Ions formed from 1 atom. The number of atoms of each element contained in a single molecule of the compound. Na 2 cro 4 cr 2 o 7.
PPT Modern Chemistry Chapter 7 Chemical Formulas and Chemical
Common chemicals and their common names. Web get faster at matching terms created by trina2017 terms in this set (48) hydrocarbons molecular compounds composed solely of carbon and hydrogen monatomic ions ions formed from a single atom stock system system of naming chemical ions and elements binary compound a compound. Valence electrons and oxidation state. Web chapter 7 practice test.
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
Web get faster at matching terms created by trina2017 terms in this set (48) hydrocarbons molecular compounds composed solely of carbon and hydrogen monatomic ions ions formed from a single atom stock system system of naming chemical ions and elements binary compound a compound. Names & formulas of chemical compounds jun 25 2023 learn and review on the go! Ions.
Chapter 7 Chemical Formulas and Chemical Compounds
Web get faster at matching terms created by trina2017 terms in this set (48) hydrocarbons molecular compounds composed solely of carbon and hydrogen monatomic ions ions formed from a single atom stock system system of naming chemical ions and elements binary compound a compound. Total number of positive and negative charges must be equal. Web modern chemistry 57 chemical bonding.
Chapter 7 Chemical Formulas and Chemical Compounds Section 2
Web chemistry quick review: B) number of atoms or ions in a compound. C) type of bond holding particles together in a compound. Web chapter 7 review : Ions formed from 1 atom.
PPT Modern Chemistry Chapter 7 Chemical Formulas & Chemical Compounds
Use quick review chemistry notes to help you learn or brush up on the subject quickly. Label each of the following statements as true or false: Represents the simplest whole number ratio of the compounds. Web learn how to name binary and polyatomic ionic compounds, and see some examples of naming these types of compounds. Web get faster at matching.
Chapter 7 Chemical Formulas and Chemical Compounds
Naming system or binary ionic compounds involves combining names of compounds. Web get faster at matching terms created by trina2017 terms in this set (48) hydrocarbons molecular compounds composed solely of carbon and hydrogen monatomic ions ions formed from a single atom stock system system of naming chemical ions and elements binary compound a compound. Chemical compounds and chemical formulas..
Chapter 7 * Chemical Formulas & Compounds
Total number of positive and negative charges must be equal. So the sulfur (“s”) is 6+. Formula mass, molar mass, and percentage composition can be calculated from the chemical formula for a compound. Naming ionic and covalent compounds. Valence electrons and oxidation state.
Names & Formulas Of Chemical Compounds Jun 25 2023 Learn And Review On The Go!
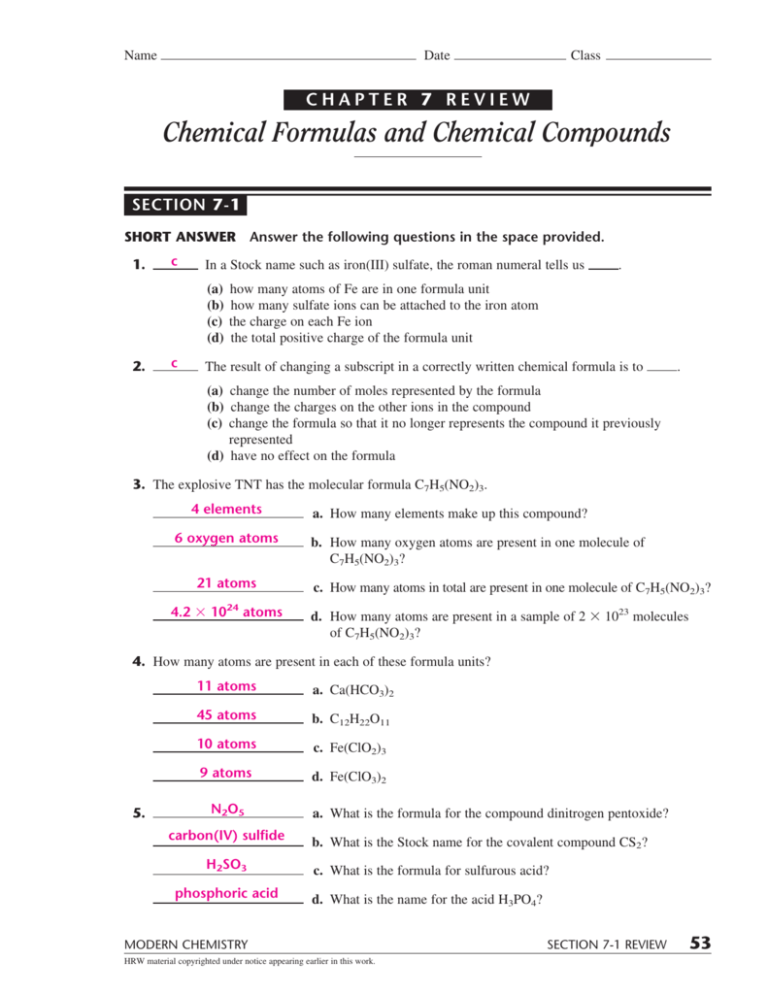
Use quick review chemistry notes to help you learn or brush up on the subject quickly. Chemical formulas and chemical compounds. Web modern chemistry 57 chemical bonding chapter 7 review chemical formulas and chemical compounds section 3 short answer answer the following questions in the space provided. Significance of a chemical formula a.
General Rules For Naming Chemical Compounds…
Web chemistry quick review: Web learn how to name binary and polyatomic ionic compounds, and see some examples of naming these types of compounds. The empirical formula for a compound shows the symbols of the elements with subscripts indicating the. Formula mass, molar mass, and percentage composition can be calculated from the chemical formula for a compound.
C) Type Of Bond Holding Particles Together In A Compound.
Ions formed from 1 atom. Relative number of atoms of each kind in a chemical formula. Web chapter 7 practice test with answer key.pdf. Na 2 cro 4 cr 2 o 7.
Total Number Of Positive And Negative Charges Must Be Equal.
6= ethane (2 carbon atoms, 6 hydrogen atoms) b. Web formula for a polyatomic ion is equal to the charge on that ion. What is the oxidation number of the atoms in these formulas? Naming system or binary ionic compounds involves combining names of compounds.