Chapter 14 Review Acids And Bases
Chapter 14 Review Acids And Bases - What are three examples of compounds that are classified as acid. Review this material, and answer the following questions: Web chemistry chapter 14 ~acids and bases test review. 1 answer an arrhenius acid is a substance which produces $h^+$ ions when dissolved in an aqueous solution. Web since acids donate hydrogen ions and bases accept hydrogen ions, it is no surprise that combining an acid with a base produces a chemical reaction. Web in chemistry, acids and bases have been defined differently by three sets of theories: What types of compounds form acidic oxides? Acids and bases of the regents chemistry. Web acids and bases are common solutions that exist everywhere. Web this chapter 14 review, section 2:
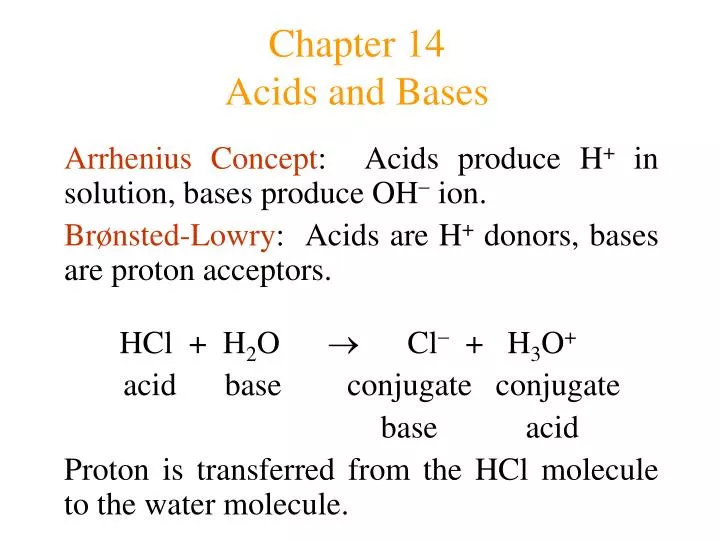
Web acids are proton donors, bases are proton acceptors. Web acids and bases are common solutions that exist everywhere. Two pages take chemistry learners on a survey of acids and bases. An indicator that turns red in acid and blue in basic. The free hydrogen ions (h +) released from the acid combine with the free hydroxide ions from the base (oh −) to form water (h 2 o) and a salt: Binary acid an acid that contains only two different elements: React with bases to produce salts and water/ 4. Conduct electricity in aqueous solutions. A molecule can be either an acid or a base depending on whether or not. Web learn test match created by ashleysanders47 terms in this set (51) acids reacts with base to form salt and water.
Starts with h+ (hydrogen ion). Binary acid an acid that contains only two different elements: An indicator that turns red in acid and blue in basic. The free hydrogen ions (h +) released from the acid combine with the free hydroxide ions from the base (oh −) to form water (h 2 o) and a salt: Web since acids donate hydrogen ions and bases accept hydrogen ions, it is no surprise that combining an acid with a base produces a chemical reaction. Web an acid that releases few hydrogen ions in aqueous solutions. Web unit 14 acids and bases test review. Web acids and bases are common solutions that exist everywhere. High schoolers write formulas and name compounds. Two pages take chemistry learners on a survey of acids and bases.
CHAPTER 15. Acids and Bases
Web unit 14 acids and bases test review. Web chemistry chapter 14 ~acids and bases test review. Web since acids donate hydrogen ions and bases accept hydrogen ions, it is no surprise that combining an acid with a base produces a chemical reaction. A quizlet set that is meant to serve as a review of all of the fundamental terms.
Chapter 14 Acids and bases
Web an acid that releases few hydrogen ions in aqueous solutions. Web in chemistry, acids and bases have been defined differently by three sets of theories: What are three examples of compounds that are classified as acid. Binary acid an acid that contains only two different elements: Buffers, titrations, and solubility equilibria.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
Web acids are proton donors, bases are proton acceptors. Web introductory chemistry acids and bases are common substances found in many every day items, from fruit juices and soft drinks to soap. Web acids and bases are common solutions that exist everywhere. A molecule can be either an acid or a base depending on whether or not. Web an acid.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
Web most acids react with solid carbonates, as in the following equation: Review this material, and answer the following questions: What types of compounds form acidic oxides? Two pages take chemistry learners on a survey of acids and bases. Web chemistry chapter 14 ~acids and bases test review.
Chapter 10 Acids and Bases
The free hydrogen ions (h +) released from the acid combine with the free hydroxide ions from the base (oh −) to form water (h 2 o) and a salt: Almost every liquid that we encounter in our daily lives consists of acidic and basic properties, with the exception of water. React with active metals/ 5. Buffers, titrations, and solubility.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
Binary acid an acid that contains only two different elements: Web bases chapter 14 big idea acids are substances that donate hydrogen ions in aqueous solutions. 1 answer an arrhenius acid is a substance which produces $h^+$ ions when dissolved in an aqueous solution. Web most acids react with solid carbonates, as in the following equation: A quizlet set that.
PPT Chapter 2 Acids & Bases PowerPoint Presentation, free download
Web unit 14 acids and bases test review. What is an acid anhydride? Starts with h+ (hydrogen ion). Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes review of acids and bases study guide has everything you need to ace quizzes, tests, and essays. Web introductory chemistry acids and bases are common substances found.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free
A quizlet set that is meant to serve as a review of all of the fundamental terms and essential concepts introduced in chapter fourteen: Web unit 14 acids and bases test review. These ap chemistry notes will cover the key topics discussed in this chapter. Web an acid that releases few hydrogen ions in aqueous solutions. Web this chapter 14.
PPT Chapter 14 Acids & Bases PowerPoint Presentation, free download
React with bases to produce salts and water/ 4. What are three examples of compounds that are classified as acid. In this unit we'll examine what the properties are of acids and bases, and. A quizlet set that is meant to serve as a review of all of the fundamental terms and essential concepts introduced in chapter fourteen: Review this.
PPT Chapter 14 Acids and Bases PowerPoint Presentation ID2145666
These ap chemistry notes will cover the key topics discussed in this chapter. Web this chapter 14 review, section 2: Two pages take chemistry learners on a survey of acids and bases. What are three examples of compounds that are classified as acid. React with active metals/ 5.
Web Since Acids Donate Hydrogen Ions And Bases Accept Hydrogen Ions, It Is No Surprise That Combining An Acid With A Base Produces A Chemical Reaction.
Web acids always have an extra h+ ion so the species with the most h's is an acid. Web this chapter 14 review, section 2: Almost every liquid that we encounter in our daily lives consists of acidic and basic properties, with the exception of water. The free hydrogen ions (h +) released from the acid combine with the free hydroxide ions from the base (oh −) to form water (h 2 o) and a salt:
Web In Chemistry, Acids And Bases Have Been Defined Differently By Three Sets Of Theories:
Web learn test match created by ashleysanders47 terms in this set (51) acids reacts with base to form salt and water. Web from a general summary to chapter summaries to explanations of famous quotes, the sparknotes review of acids and bases study guide has everything you need to ace quizzes, tests, and essays. High schoolers write formulas and name compounds. Web most acids react with solid carbonates, as in the following equation:
Starts With H+ (Hydrogen Ion).
What types of compounds form acidic oxides? What is an acid anhydride? Acids and bases of the regents chemistry. An acid and base that are related by removing or adding a single hydrogen ion.
An Indicator That Turns Red In Acid And Blue In Basic.
A molecule can be either an acid or a base depending on whether or not. What are three examples of compounds that are classified as acid. In this unit we'll examine what the properties are of acids and bases, and. Buffers, titrations, and solubility equilibria.