Carbon Dioxide Dissolves In Water To Form
Carbon Dioxide Dissolves In Water To Form - Web in reaction , gaseous carbon dioxide is dissolved in water, reacting to form carbonic acid. Estimate the thermodynamic equilibrium constant for this reaction using. Web carbon dioxide dissolves in water to form carbonic acid. Volume of solution does not change on dissolution of. Web expert answer transcribed image text: Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. 30% of all carbon dioxide is dissolved in water. Web answer (1 of 2): H 2co 3 is a. Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (.
Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Carbon dioxide dissolves in water to form carbonic acid. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Most of it gets dissolved. Web in reaction , gaseous carbon dioxide is dissolved in water, reacting to form carbonic acid. Web when carbon dioxide dissolves in water only some molecules of it will react with water. Co2 dissolves in water to form carbonic acid: H 2co 3 is a. Web cold water can dissolve more carbon dioxide than warm water. The concentration of dissolved carbon dioxide therefore.
Web cold water can dissolve more carbon dioxide than warm water. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Most of it gets dissolved. Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Web expert answer transcribed image text: Web in reaction , gaseous carbon dioxide is dissolved in water, reacting to form carbonic acid. Volume of solution does not change on dissolution of. Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Most of it gets dissolved, co 2 remains loosely hydrated. This is due to its polar bonds, and its reaction with water.
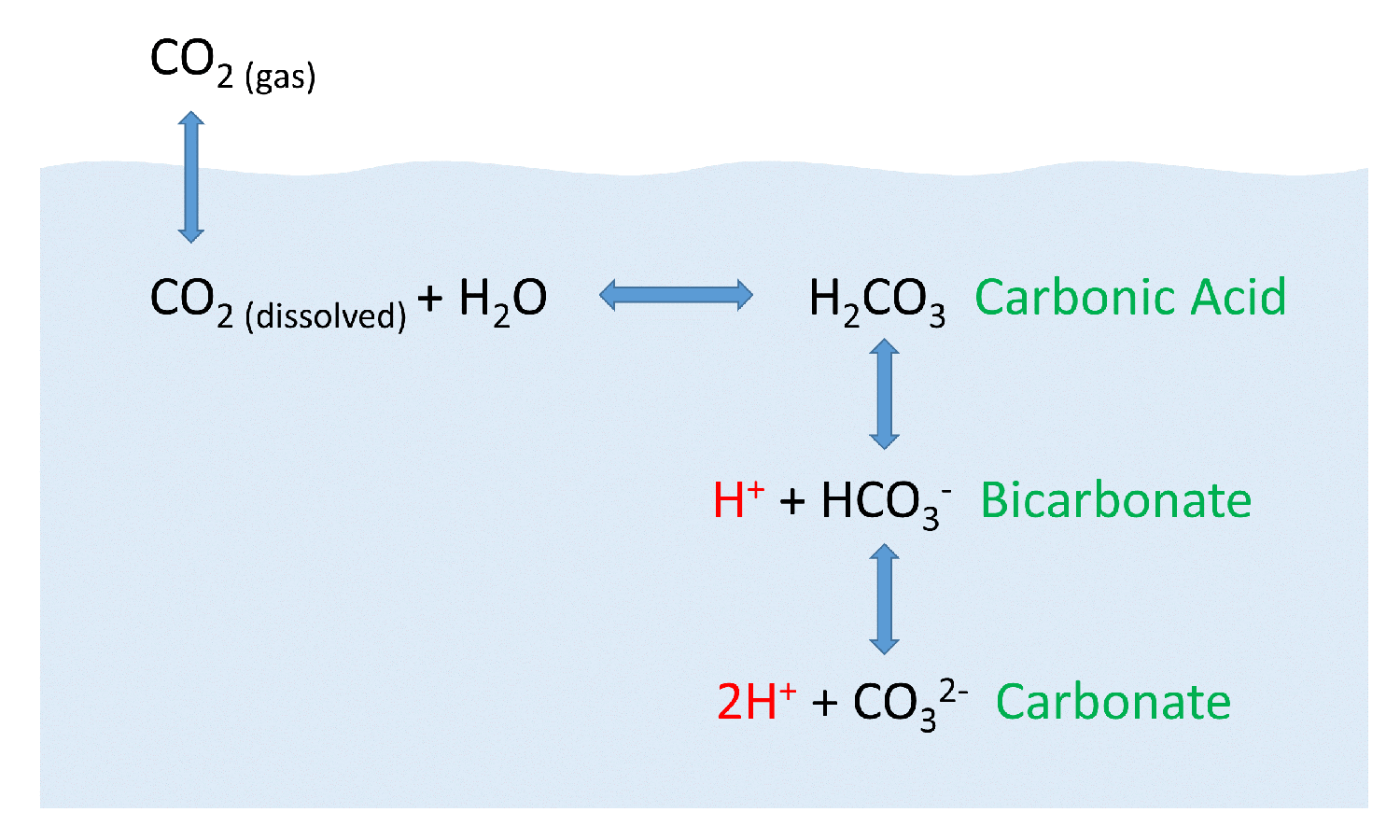
Summary of the reactions between carbon dioxide (CO2) with water (H2O
It reacts with water ( h 2 o) and forms carbonic acid ( h 2. Web carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. The bicarbonate ions can further. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Web answer (1 of 2):
Carbon Cycle Jeopardy Template
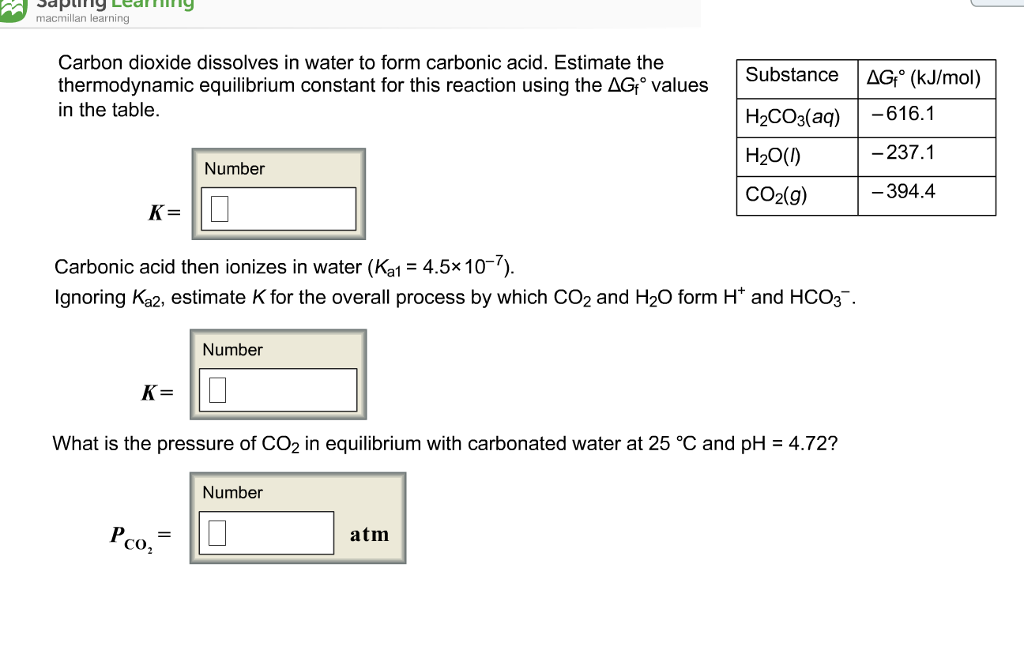
Dissolved co2 satisfies the equilibrium equation. Reaction of carbon dioxide with water is neglected. Web expert answer transcribed image text: H 2co 3 is a. Estimate the thermodynamic equilibrium constanst (k) for this reaction (delta gf values:
😂 When carbon dioxide dissolves in water. What does carbon dioxide form
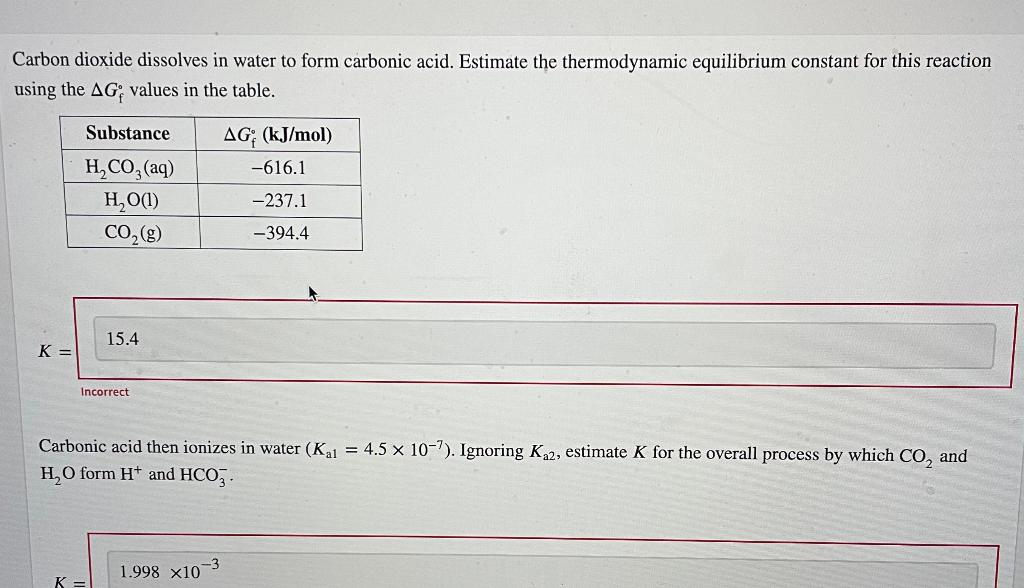
Dissolved co, satisfies the equilibrium. Estimate the thermodynamic equilibrium constant for this reaction using the ag, values in the table. Web carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Carbon dioxide dissolves.
5 Important Sources of Greenhouse Gases
(carbonic acid), which is a weak acid since its ionization in water is incomplete. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. This is due to its polar bonds, and its reaction with water. Reaction of carbon dioxide with water is neglected. Estimate the thermodynamic equilibrium constant for this reaction.
Understanding the Science of Ocean and Coastal Acidification Ocean
Web answer (1 of 2): Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Web expert answer transcribed image text: Web correct option is a) when co 2 dissolves in h 2o, only some molecules react with h 2) to form h 2co 3. Hydrogen ions dissociate from the carbonic acid,.
The reaction between carbon dioxide and water Experiment RSC Education
Web carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Web correct option is a) when co 2 dissolves in h 2o, only some molecules react with h 2) to form h 2co 3. Volume of solution does not change on dissolution of. H 2co 3 is a. The concentration of dissolved carbon dioxide therefore.
Water could modulate the activity and selectivity of carbon dioxide
Most of it gets dissolved. This is due to its polar bonds, and its reaction with water. Estimate the thermodynamic equilibrium constant for this reaction using. Estimate the thermodynamic equilibrium constanst (k) for this reaction (delta gf values: Web carbon dioxide dissolves in water to form carbonic acid.
Transforming Carbon Dioxide to Into Fuel More Efficiently With a Water
Carbon dioxide dissolves in water to form carbonic acid. Holding 100ml of water (ebkare)________________2. Dissolved co, satisfies the equilibrium. Dissolving anything is a physical change. Web chemistry chemistry questions and answers carbon dioxide dissolves in water to form carbonic acid.
Solved Carbon dioxide dissolves in water to form carbonic
The concentration of dissolved carbon dioxide therefore. Dissolving anything is a physical change. Web in reaction , gaseous carbon dioxide is dissolved in water, reacting to form carbonic acid. It reacts with water ( h 2 o) and forms carbonic acid ( h 2. Estimate the thermodynamic equilibrium constant for this reaction using.
Water Analysis Dissolved Carbon Dioxide Advanced BioTech
Most of it gets dissolved, co 2 remains loosely hydrated. Web carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Web co2 is quite soluble in water: Web cold water can dissolve more carbon dioxide than warm water. Estimate the thermodynamic equilibrium constant for this reaction using.
Volume Of Solution Does Not Change On Dissolution Of.
Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Estimate the thermodynamic equilibrium constant for this reaction using the ag, values in the table. Web co2 is quite soluble in water: Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3.
This Is Due To Its Polar Bonds, And Its Reaction With Water.
Web cold water can dissolve more carbon dioxide than warm water. Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Web correct option is a) when co 2 dissolves in h 2o, only some molecules react with h 2) to form h 2co 3. The bicarbonate ions can further.
Web Expert Answer Transcribed Image Text:
Web carbon dioxide dissolves in water to form carbonic acid. Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Vapour pressure of water is negligible. Dissolved co2 satisfies the equilibrium equation.
Web Answer (1 Of 2):
Web carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. Estimate the thermodynamic equilibrium constant for this reaction using. Web chemistry chemistry questions and answers carbon dioxide dissolves in water to form carbonic acid. Most of it gets dissolved.