Atoms Form Bonds To
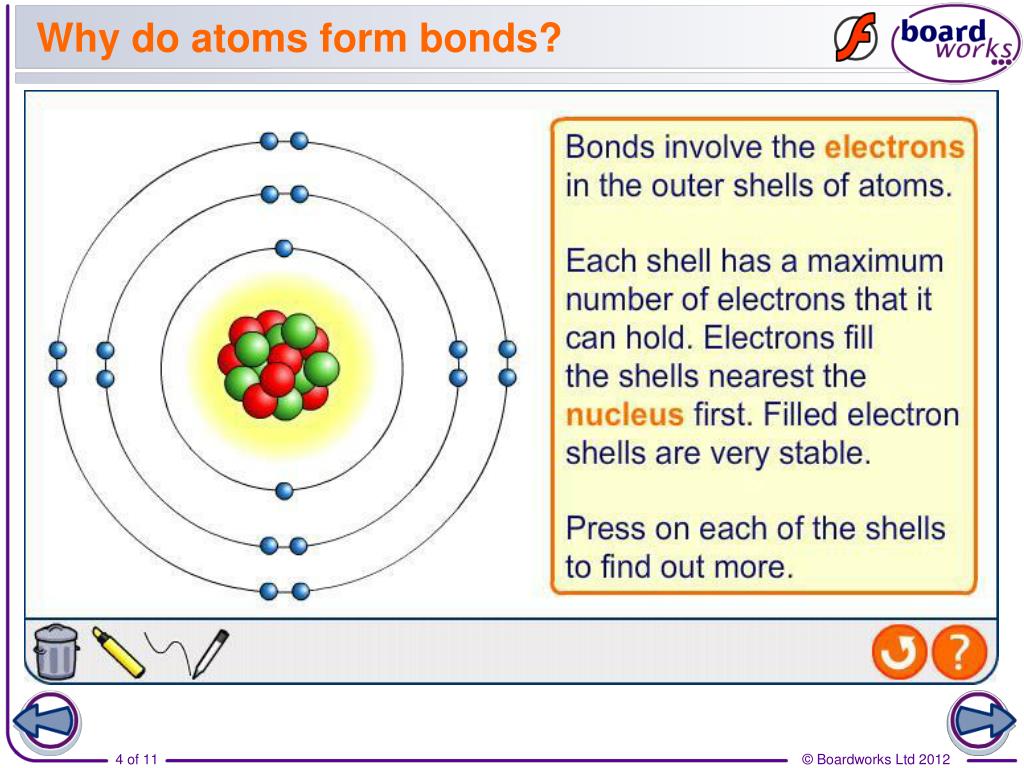
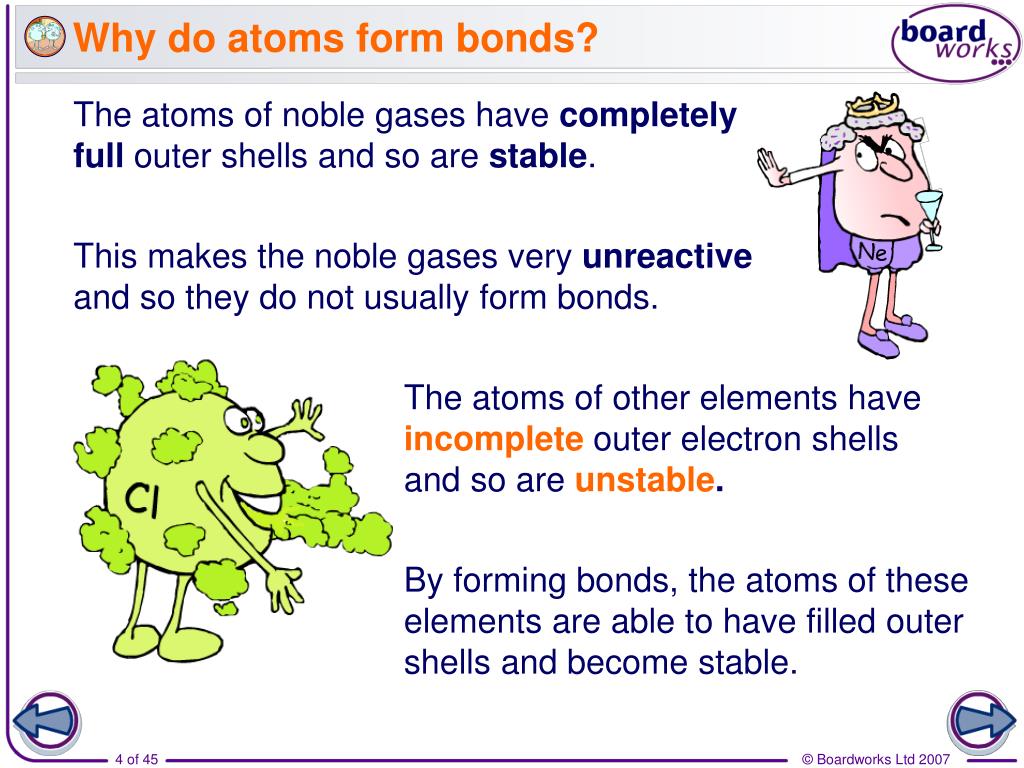
Atoms Form Bonds To - Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The bond may result from the electrostatic. The combination of multiple atoms, or chemical bonding, forms molecules. A polar covalent bond is a covalent. Web in this model, covalent bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. There are several types of bond. Protons and neutrons compose the positively charged nucleus whilst electrons orbit in orbitals (or. Chemical bonds are formed when electrons in different atoms. Web with the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). So, how do atoms make friends?
When two oxygen atoms bond, they become a molecule. Web atoms are individual units made up of protons, neutrons, and electrons. An atom can go any distance to complete its octet!. For example, water, ( h 2 o ), has two covalent bonds between a single oxygen atom and. A polar covalent bond is a covalent. Web the number of bonds an atom can form depends on that atom's valence electrons. Web with the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). There are several types of bond. Web in this model, covalent bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or a.
So, in order to fill these. Here are some examples of molecules with covalent bonds. There are several types of bond. The simplest answer is with their electrons and, more specifically, with. Chemical bonds are formed when electrons in different atoms. Web with the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). The bond may result from the electrostatic. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. The combination of multiple atoms, or chemical bonding, forms molecules. Web when atoms get together to make friends, it is called chemical bonding.
Solved 1. Why do atoms form bonds? Explain the difference
An atom can go any distance to complete its octet!. Web there are three kinds of chemical bonds: Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Elements are made of protons, neutrons and electrons. Web two different atoms can also share electrons and form covalent bonds.
Why do atoms form bonds? Video] O Level Secondary Chemistry
So, how do atoms make friends? Chemical bonds are formed when electrons in different atoms. Web there are three kinds of chemical bonds: Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Web atoms are individual units made up of protons, neutrons, and electrons.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
The combination of multiple atoms, or chemical bonding, forms molecules. Web answer (1 of 16): So, how do atoms make friends? Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h.
Solved What Is The Maximum Number Of Bonds That The Atom
Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Web in this model, covalent bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. Web in a metallic bond, each metal atom is surrounded by lots.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web two different atoms can also share electrons and form covalent bonds. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Elements are made of protons, neutrons and electrons. Web the number of bonds an atom can form depends on that atom's valence electrons. The bond may.
PPT Ions PowerPoint Presentation, free download ID6738771
Web in a metallic bond, each metal atom is surrounded by lots of other metal atoms, and they all share their valence electrons. Web in this model, covalent bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. Web in an ionic bond, the atoms are bound together by.
Why do atoms form bonds? YouTube
Web two different atoms can also share electrons and form covalent bonds. Web in an ionic bond, the atoms are bound together by the electrostatic forces in the attraction between ions of opposite charge. When two oxygen atoms bond, they become a molecule. So, in order to fill these. Web atoms can join together by forming a chemical bond, which.
Student Exploration Ionic Bonds Answer Key Quizlet / Ionic Bonds Gizmo
Ionic bonds usually occur between metal and. The bond may result from the electrostatic. Here are some examples of molecules with covalent bonds. Web atoms are individual units made up of protons, neutrons, and electrons. So, how do atoms make friends?
thinkbiggerdesigns Why Do Atoms Ions
When two oxygen atoms bond, they become a molecule. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or a. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called.
Biochemistry Honors
Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Here are some examples of molecules with covalent bonds. Web in this model, covalent bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. Web when both.
An Atom Can Go Any Distance To Complete Its Octet!.
Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. The simplest answer is with their electrons and, more specifically, with. There are several types of bond. Here are some examples of molecules with covalent bonds.
Web Answer (1 Of 16):
Web in a metallic bond, each metal atom is surrounded by lots of other metal atoms, and they all share their valence electrons. Web in an ionic bond, the atoms are bound together by the electrostatic forces in the attraction between ions of opposite charge. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or a. Web there are three kinds of chemical bonds:
Web The Number Of Bonds An Atom Can Form Depends On That Atom's Valence Electrons.
Web atoms are individual units made up of protons, neutrons, and electrons. Protons and neutrons compose the positively charged nucleus whilst electrons orbit in orbitals (or. Ionic bonds usually occur between metal and. A polar covalent bond is a covalent.
Web A Chemical Bond Is A Lasting Attraction Between Atoms Or Ions That Enables The Formation Of Molecules, Crystals, And Other Structures.
For example, water, ( h 2 o ), has two covalent bonds between a single oxygen atom and. Elements are made of protons, neutrons and electrons. Web with the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). The combination of multiple atoms, or chemical bonding, forms molecules.

![Why do atoms form bonds? Video] O Level Secondary Chemistry](https://icandochemistry942105908.files.wordpress.com/2021/06/thumb-1.png?w=1200)